- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Murine Alveolar Type II Epithelial Cells
Published: Vol 7, Iss 10, May 20, 2017 DOI: 10.21769/BioProtoc.2288 Views: 13116
Reviewed by: HongLok LungJason A. NeidlemanShahzada Khan

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Soft Agar Colony Formation Assay as a Hallmark of Carcinogenesis
Feng Du [...] Daiming Fan
Jun 20, 2017 30592 Views

A Fast and Reliable Method to Generate Pure, Single Cell-derived Clones of Mammalian Cells
Zhe Han [...] Varun Kumar
Aug 20, 2022 4105 Views
Abstract
We have optimized a protocol for isolation of alveolar type II epithelial cells from mouse lung. Lung cell suspensions are prepared by intratracheal instillation of dispase and agarose followed by mechanical disaggregation of the lungs. Alveolar type II epithelial cells are purified from these lung cell suspensions through magnetic-based negative selection using a Biotin-antibody, Streptavidin-MicroBeads system. The purified alveolar type II epithelial cells can be cultured and maintained on fibronectin-coated plates in DMEM with 10% FBS. This protocol enables specific investigation of alveolar type II epithelial cells at molecular and cellular levels and provides an important tool to investigate in vitro the mechanisms underlying lung pathogenesis.
Keywords: Alveolar type II epithelial cellsBackground
Alveolar type II epithelial cells play critical roles in alveolar integrity maintenance, surfactant protein synthesis and secretion, and defense against pulmonary infection of bacteria and viruses. Recent studies using mouse lung cancer models have proven that alveolar type II epithelial cells are a key cell of origin of adenoma/adenocarcinoma induced by chemical carcinogens and oncogenic mutations (Qu et al., 2015; Zhou et al., 2015 and 2017). To further expand our understanding of the role of alveolar type II epithelial cells in lung pathogenesis in vivo, isolation of alveolar type II epithelial cells is needed to allow for a precise mechanism analysis in vitro. Based on previous studies (Corti et al., 1996; Rice et al., 2002), a modified method was used in our laboratory to isolate highly purified, viable and culturable alveolar type II epithelial cells from mice (Zhou et al., 2015; Sun et al., 2016).
Materials and Reagents
- Needles (BD, catalog number: 305167 ) or tapes
- 10 ml syringe (BD, catalog number: 309604 )
- 27 gauge needle (BD, catalog number: 305109 )
- Nylon string (Dynarex, catalog number: 3243 )
- 22 G x 1” Exel Safelet Catheter (Exel International, catalog number: 26746 )
- 1 ml syringe (BD, catalog number: 309659 )
- 15 ml tubes (VWR, catalog number: 89039-666 )
- 60 mm non-coated cell culture dish (Greiner Bio One International, catalog number: 628160 )
- Cell strainer (70 µm) (Fisher Scientific, catalog number: 22-363-548 )
- Cell strainer (40 µm) (Fisher Scientific, catalog number: 22-363-547 )
- Nylon mesh (25 µm) (ELKO filtering, catalog number: 03-25/19 )
- MS column (Miltenyi Biotec, catalog number: 130-042-201 )
- Fibronectin-coated plate (Corning, catalog number: 354402 )
- Mice (THE JACKSON LABORATORY)
- 70% ethanol (Decon Labs, catalog number: 2701 )
- Dispase (1 mg/ml dissolved in PBS) (Roche Diagnostics, catalog number: 4942078001 )
- 1% low melting point agarose (Dissolved in PBS, autoclaved, aliquoted and stored at 4 °C) (Lonza, catalog number: 50100 )
- DMEM (Lonza, catalog number: 12-604F )
- DNase I (Roche Diagnostics, catalog number: 10104159001 )
- Biotinylated anti-CD45 (Miltenyi Biotec, catalog number: 130-101-952 )
- Biotinylated anti-CD16/CD32 (Miltenyi Biotec, catalog number: 130-101-895 )
- Streptavidin MicroBeads (Miltenyi Biotec, catalog number: 130-048-101 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437028 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9625 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Disodium hydrogen phosphate heptahydrate (Na2HPO4·7H2O) (Fisher Scientific, catalog number: BP331-500 )
- Potassium phosphate monobasic (KH2PO4) (Acros Organics, catalog number: 205925000 )
- Bovine serum albumin (BSA) (MP Biomedicals, catalog number: 199898 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E26282-500G )
Note: This product has been discontinued. - Penicillin-streptomycin (Lonza, catalog number: 17-602E )
- Phosphate buffered saline (PBS) (see Recipes)
- Labeling buffer (see Recipes)
Equipment
- CO2 chamber
- Biosafety cabinet
- Styrofoam board
- Forceps (Roboz Surgical Instrument, catalog number: RS-5135 )
- Scissors (Roboz Surgical Instrument, catalog number: RS-6802 )
- Water bath incubator (Thermo Fisher Scientific, Thermo ScientificTM, model: BarnsteadTM 18000A-1CE )
- Shaker (Thermo Fisher Scientific, Thermo ScientificTM, model: BarnsteadTM 2314 )
- Centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: IEC CL40R , catalog number: 11210927)
- MACS MultiStand (Miltenyi Biotec, catalog number: 130-042-303 )
- Hemocytometer (Hausser Scientific, catalog number: 3110 )
Procedure
- Preparation of crude single lung cell suspension
- Sacrifice a mouse by CO2 inhalation in a CO2 chamber.
- Bring the mouse to a biosafety cabinet.
- Dampen the mouse with 70% ethanol.
- Place the mouse front side up on dissecting Styrofoam board and fix the arms and legs with needles or tape.
- Use scissors to make incision in the skin from abdomen to neck, and tear skin with forceps to expose thoracic cage and neck.
- Gently remove the muscle around the neck to expose the trachea.
- Carefully cut the ribs to expose the heart and lungs.
- Perfuse the lung with 0.9% NaCl, using a 10 ml syringe fitted with a 27 gauge needle, through the right ventricle of heart until it is visually free of blood.
- Use forceps to put a ~10 cm-long nylon string under the trachea.
- Insert the 22 G x 1” Exel Safelet Catheter into the trachea, remove stylet hub, and tie a knot with a nylon string to secure catheter and trachea together firmly.
- Slowly inject 2 ml of dispase into the lung, and allow the lung to collapse for 5 min.
- Quickly load a 1 ml syringe with 0.5 ml of 1% low melting point agarose (brought from 4 °C storage, thawed in a 70 °C water bath, and then kept in a 45 °C water bath in a melted status), replace the dispase syringe with the agarose-containing syringe, and gently infuse the lung with the loaded agarose. Leave the syringe in place, and immediately cover the lung with ice and incubate for 2 min to promote the solidification of agarose.
- Remove the lung from animal to a 15 ml tube containing 2 ml of dispase, incubate for 45 min at room temperature on a shaker.
Note: Shaking helps dispase digestion and cell release, but the exact speed of shaking is not critical. - Transfer the lung to 7 ml of room-temperature DMEM with 0.01% DNase I in a 60 mm non-coated cell culture dish. The digested tissue is carefully teased from bronchi with scissors and forceps.
- The resulting cell suspension is successively filtered through 70 µm and 40 µm cell strainers, and then 25 µm nylon mesh.
- Sacrifice a mouse by CO2 inhalation in a CO2 chamber.
- Magnetic labeling
- Count the filtered cells by a hemocytometer, centrifuge cell suspension at 300 x g for 10 min at 4 °C, and then resuspend in 400 µl of labeling buffer per 107 cells.
- Add 5 µl of each biotinylated anti-CD45 and biotinylated anti-CD16/CD32, mix well and incubate for 10 min at 4 °C.
- Wash cells with 1 ml of labeling buffer and centrifuge at 300 x g for 10 min at 4 °C.
- Resuspend cell pellet in 90 µl of labeling buffer per 107 cells.
Note: In steps B4-B8, if working with fewer than 107 cells, use the same volume as indicated. - Add 10 µl of Streptavidin MicroBeads per 107 cells.
- Mix well and incubate for 15 min at 4 °C.
- Wash cells with 1 ml of labeling buffer and centrifuge at 300 x g for 10 min at 4 °C.
- Resuspend the cell pellet in 500 µl of labeling buffer.
- Count the filtered cells by a hemocytometer, centrifuge cell suspension at 300 x g for 10 min at 4 °C, and then resuspend in 400 µl of labeling buffer per 107 cells.
- Magnetic separation
- Place an MS column in the magnetic field of a suitable MACS MultiStand.
- Prepare column by rinsing with 0.5 ml of labeling buffer.
- Apply cell suspension onto the column. Collect flow-through containing unlabelled cells, representing the alveolar type II cells.
- Wash column 3 times with 0.5 ml of labeling buffer each. Collect unlabelled cells that pass-through, and combine with the effluent from step C3.
- Place an MS column in the magnetic field of a suitable MACS MultiStand.
- Alveolar type II cell culture
- The combined effluents are centrifuged at 300 x g for 10 min at 4 °C. The cell pellet is resuspended to 106 cells per ml in DMEM with 10% FBS, and cultured for 12 h in non-coated cell culture plate in a humidified, 5% CO2 incubator at 37 °C to remove residual mesenchymal cells attached to plate.
Note: Generally, 4-5 million cells will be retrieved per mouse lung, and ~4 ml medium will be used to resuspend the isolated cells at 106 cells per ml. - Cell suspensions with unattached cells (mainly alveolar type II epithelial cells) are gently collected and centrifuged at 300 x g for 10 min at 4 °C. The pellet is resuspended in DMEM with 10% FBS and cultured on a fibronectin-coated plate. The typical morphology of alveolar type II epithelial cells is shown in Figure 1. Meanwhile, genomic sequencing of cultured alveolar type II epithelial cells is performed to detect the mutations of K-Ras gene (Figure 2).
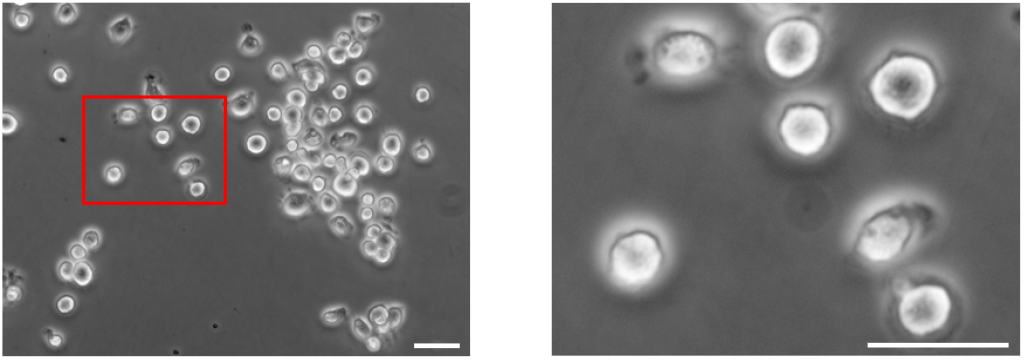
Figure 1. Isolated murine alveolar type II epithelial cells cultured in complete DMEM on fibronectin-coated plate for 5 days. Image on the right is the magnification of the red-box area on the left. Scale bars = 20 µm.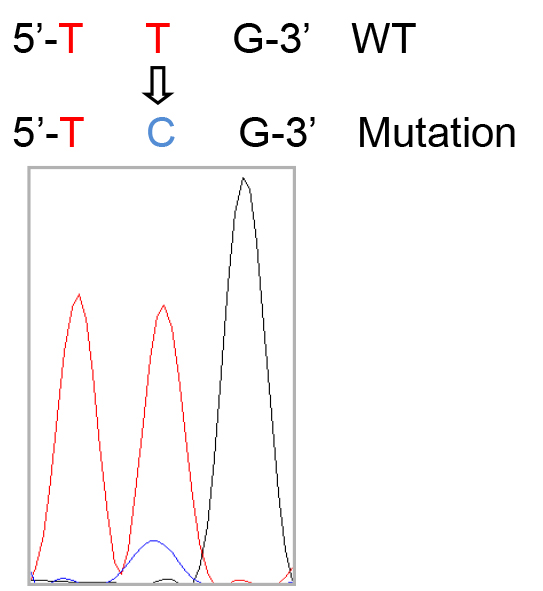
Figure 2. The codon 61 mutation of K-Ras gene in alveolar type II epithelial cell. Q61 codon: CAA (reverse complementary: TTG).
- The combined effluents are centrifuged at 300 x g for 10 min at 4 °C. The cell pellet is resuspended to 106 cells per ml in DMEM with 10% FBS, and cultured for 12 h in non-coated cell culture plate in a humidified, 5% CO2 incubator at 37 °C to remove residual mesenchymal cells attached to plate.
Data analysis
- Morphology of isolated alveolar type II epithelial cells.
- Genome DNA is extracted from isolated alveolar type II epithelial cells from urethane-treated mice. Then the exon 3 of K-Ras gene is PCR-amplified and subsequently sequenced to detect codon 61 mutations.
Notes
- Injection of agarose helps push dispase solution deep into alveoli. The solidified agarose can prevent the reflux of dispase solution, and also reduce the contamination of cells from bronchi, such as Clara cells.
- If the isolated alveolar type II epithelial cells are to be used for culture, carry out all steps in sterile conditions with autoclaved dissection tools.
- Using this protocol, about 4-5 x 106 alveolar type II epithelial cells per mouse can be retrieved. The isolated cells can be cultured and maintained for at least 8 days. It is better to use freshly isolated/cultured alveolar type II epithelial cells. Freezing of the cells for storage is not recommended.
Recipes
- Phosphate-buffered saline (PBS) (1 L)
8.0 g NaCl
0.2 g KCl
1.15 g Na2HPO4·7H2O
0.2 g KH2PO4
Adjust to pH 7.4 - Labeling buffer
PBS, pH 7.2
0.5% BSA
2 mM EDTA
Acknowledgments
The authors thank the members in Xiao-Qu lab for helpful discussion. This study was supported in part by the National Institute of Health (NIH)/National Cancer Institute (NCI) grants R01 CA172090, R21 CA175252, P50 CA090440, P30 CA047904, as well as the American Lung Association (ALA) Lung Cancer Discovery Award LCD 259111 and American Cancer Society (ACS) Fellowship Award PF-12081-01-TBG.
References
- Corti, M., Brody, A. R. and Harrison, J. H. (1996). Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 14(4): 309-315.
- Qu Z., Sun F., Zhou J., Li L., Shapiro S. D., Xiao G. (2015). Interleukin-6 prevents the initiation but enhances the progression of lung cancer. Cancer Res 75(16): 3209-15.
- Rice, W. R., Conkright, J. J., Na, C. L., Ikegami, M., Shannon, J. M. and Weaver, T. E. (2002). Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 283(2): L256-264.
- Sun, F., Qu, Z., Xiao, Y., Zhou, J., Burns, T. F., Stabile, L. P., Siegfried, J. M. and Xiao, G. (2016). NF-κB1 p105 suppresses lung tumorigenesis through the Tpl2 kinase but independently of its NF-κB function. Oncogene 35(18): 2299-2310.
- Zhou J., Qu Z., Sun F., Han L., Li L., Yan S., Stabile L. P. , Chen L. F. , Siegfried J. M., Xiao G.. (2017). Myeloid STAT3 promotes lung tumorigenesis by transforming tumor immunosurveillance into tumor-promoting inflammation. Cancer Immunol Res 5(3): 257-268.
- Zhou, J., Qu, Z., Yan, S., Sun, F., Whitsett, J. A., Shapiro, S. D. and Xiao, G. (2015). Differential roles of STAT3 in the initiation and growth of lung cancer. Oncogene 34(29): 3804-3814.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sun, F., Xiao, G. and Qu, Z. (2017). Isolation of Murine Alveolar Type II Epithelial Cells. Bio-protocol 7(10): e2288. DOI: 10.21769/BioProtoc.2288.
Category
Cancer Biology > General technique > Cell biology assays > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










