- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Simple Spectroscopic Determination of Nitrate, Nitrite, and Ammonium in Arabidopsis thaliana
Published: Vol 7, Iss 10, May 20, 2017 DOI: 10.21769/BioProtoc.2280 Views: 20668
Reviewed by: Dennis NürnbergZhanwu DaiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vitro Colorimetric Method to Measure Plant Glutamate Dehydrogenase Enzyme Activity
Asier Sarasketa [...] Daniel Marino
Aug 20, 2015 12068 Views

A Simple and Rapid Assay for Measuring Phytoalexin Pisatin, an Indicator of Plant Defense Response in Pea (Pisum sativum L.)
Lee A. Hadwiger and Kiwamu Tanaka
Jul 5, 2017 8068 Views

In vivo and in vitro 31P-NMR Study of the Phosphate Transport and Polyphosphate Metabolism in Hebeloma cylindrosporum in Response to Plant Roots Signals
Christine Le Guernevé [...] Hervé Quiquampoix
Aug 20, 2018 6671 Views
Abstract
Plants use nitrate, nitrite, and ammonium as inorganic nitrogen (N) sources. These N compounds are included in plant tissues at various concentrations depending on the balance between their uptake and assimilation. Thus, the contents of nitrate, nitrite, and ammonium are physiological indicators of plant N economy. Here, we describe a protocol for measurement of these inorganic N species in A. thaliana shoots or roots.
Keywords: Arabidopsis thalianaBackground
Determination of inorganic N content is important for predicting the ability of a plant to uptake and assimilate N. Researchers often use techniques requiring expensive equipment, such as high-performance liquid chromatography (HPLC), for these measurements. The present protocol is based on versatile spectrometry with cheap reagents, making it practicable for many researchers. Nitration of salicylic acid by nitrate occurs under acidic conditions, and subsequent addition of an alkaline solution results in a yellow complex. Under acidic conditions, nitrite reacts with sulfanilamide to produce a diazonium compound that undergoes diazocoupling with N-(1-naphthyl)ethylenediamine to form a pink azo compound. Ammonium can be determined as a blue indophenol derivative under the catalytic influence of a nitroprusside salt. Each experimental procedure is easy, rapid, and simple.
Materials and Reagents
- 0.1-10 µl pipette tips (NIPPON Genetics, catalog number: 30470 )
- 1-200 µl pipette tips (NIPPON Genetics, catalog number: 30430 )
- 100-1,000 µl pipette tips (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 111-N-Q )
- 2.0 ml microtube with O-ring (SARSTEDT, catalog number: 72.693 )
- 1.5 ml microtube (WATSON, catalog number: 131-5155C )
- Assay plate (Iwaki, catalog number: 3881-096 )
- Gloves and eye protection
- Arabidopsis thaliana ecotype Col-0
- Ultrapure water (Milli-Q, Millipore, 18 MΩ cm)
- Liquid N2
- Sulfuric acid (Wako Pure Chemical Industries, catalog number: 192-04696 )
- Salicylic acid (Wako Pure Chemical Industries, catalog number: 196-14861 )
- Sodium hydroxide (Wako Pure Chemical Industries, catalog number: 198-13765 )
- Potassium nitrate (Wako Pure Chemical Industries, catalog number: 160-04035 )
- Reaction reagent 1 (see Recipes)
- Reaction reagent 2 (see Recipes)
Equipment
- Pipettes (Nichiryo, model: Nichipet EX II )
- Heat block (TAITEC, model: DTU-1B )
- Refrigerated centrifuge (TOMY DIGITAL BIOLOGY, model: MX-300 )
- Vortex mixer (Scientific Industries, catalog number: SI-0236 )
- Multimode plate reader (PerkinElmer, model: EnSpire® 2300 )
Note: Versatile spectrophotometers are practicable. - Spectrophotometer (Shimadzu, model: UV-1650PC )
Procedure
- Extraction of nitrate from A. thaliana shoots or roots
- Excise and weigh fresh shoots or roots, put them into a 2 ml microtube with an O-ring, and freeze them in liquid N2.
Note: Samples can be stored at -80 °C before use. We usually use 10-50 mg of samples (fresh weight) for nitrate measurements. Nitrate is extractable from the samples with or without being powdered in liquid N2 (Xu et al., 2016). We routinely use whole shoots or roots as they are for the extraction. - Add 10 vols. of ultrapure water.
Note: The volume of water added is determined based on the assumption that the pellet derived from 1 mg fresh weight has a volume of about 1 µl. Addition of water preheated at 80 °C denatures nitrate reductase more quickly, avoiding nitrate metabolism during extraction. The whole sample should be submerged in water. - Incubate at 100 °C for 20 min with the lid closed.
Note: The tubes should be shaken by hand or vortexed every 5 min for complete extraction. - Centrifuge the cooled tubes at 20,400 x g for 10 min at room temperature. Use the supernatants for nitrate determination.
Note: Supernatants can be stored at -80 °C before use.
- Excise and weigh fresh shoots or roots, put them into a 2 ml microtube with an O-ring, and freeze them in liquid N2.
- Determination of nitrate from A. thaliana shoots or roots
- Add 40 µl of reaction reagent 1 (see Recipes) to a 1.5 ml microtube to determine the apparent nitrate concentration.
- Add 40 µl of sulfuric acid instead of reaction reagent 1 to another 1.5 ml microtube to determine the nonspecific background concentration.
- Add 10 µl of supernatant.
Note: Supernatants are often sticky. We usually attach the supernatant to the wall of the microtube. - Spin down the contents and vortex them thoroughly.
- Incubate at room temperature for 20 min.
Note: The incubation temperature is usually maintained at 20-25 °C. - Gently add 1 ml of reaction reagent 2 (see Recipes), and vortex until the contents become clear.
Note: This neutralization process generates heat. The obtained yellow product is stable for 48 h in the dark. - Measure the absorbance at 410 nm using a spectrophotometer.
- Add 40 µl of reaction reagent 1 (see Recipes) to a 1.5 ml microtube to determine the apparent nitrate concentration.
- Preparation of nitrate standard curve
- Prepare a nitrate dilution series using potassium nitrate (0, 0.125, 0.25, 0.5, 1, 2, 4, and 8 mM) in ultrapure water.
Note: The dilution series can be stored at -20 °C. - Add 40 µl of reaction reagent 1 to a 1.5 ml microtube.
- Add 10 µl of a standard solution.
- Spin down the contents and vortex them thoroughly.
- Incubate at room temperature for 20 min.
- Gently add 1 ml of reaction reagent 2, and vortex until the contents become clear.
- Measure the absorbance at 410 nm using the spectrophotometer and construct a standard curve (Figure 1). A picture of a representative nitrate dilution series is shown in Figure 2.
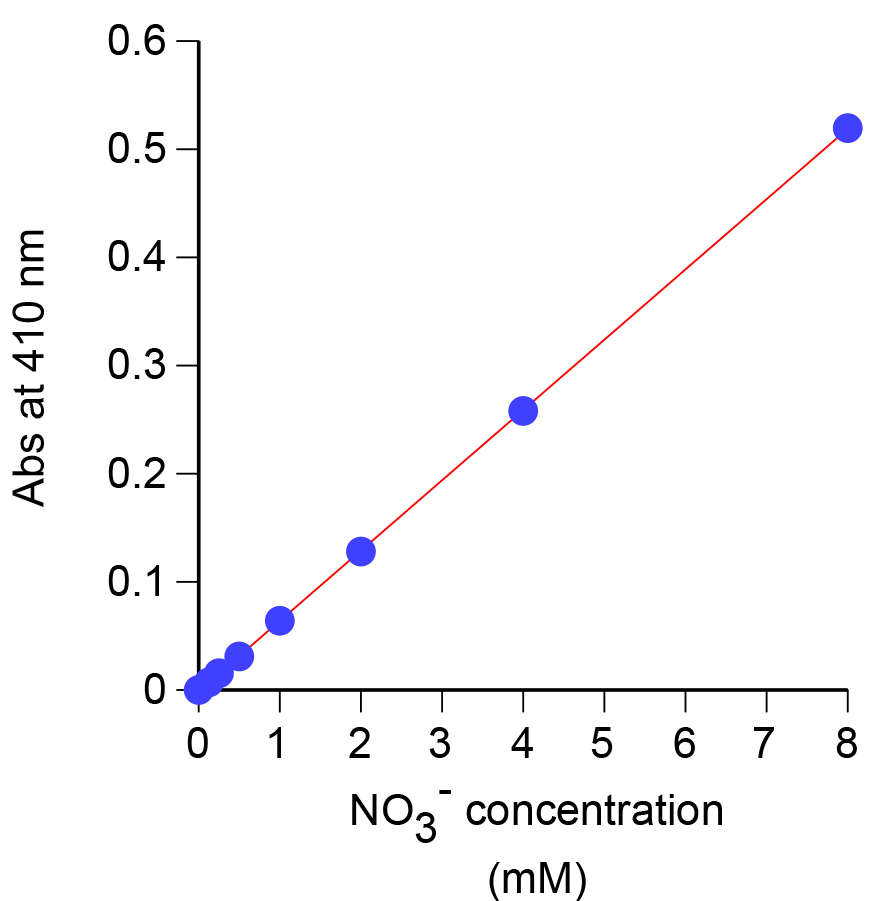
Figure 1. Standard curve generated using the absorbance at 410 nm for known concentrations of nitrate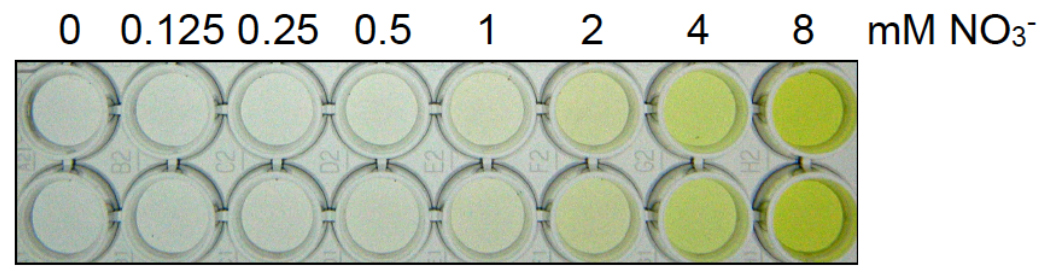
Figure 2. Picture of a representative nitrate dilution series
- Prepare a nitrate dilution series using potassium nitrate (0, 0.125, 0.25, 0.5, 1, 2, 4, and 8 mM) in ultrapure water.
Data analysis
- A typical nitrate standard curve is shown in Figure 1. From the curve, the nitrate concentration (mM) can be determined using the formula: (Abs410 - intercept)/slope.
- Calculate the apparent nitrate concentration (mM) of the supernatant using the standard curve.
- Calculate the nonspecific background concentration (mM) of the supernatant using the standard curve.
- Calculate the true nitrate concentration (mM) of the supernatant by subtracting the nonspecific value from the apparent value.
- Determine the nitrate content of the sample (µmol g-1 fresh weight) using the formula: [true nitrate concentration (mM)] x [extracted volume (ml)]/[fresh weight (g)].
Notes
Sulfuric acid, salicylic acid, and sodium hydroxide are very dangerous and harmful for humans and the environment. Wear gloves and eye protection, and discard waste liquid properly. Please make sure that RISK assessments are carried out before conducting experiments. At least two technical replicates should be obtained for each sample and standard. More than five biological replicates are desirable to obtain reliable data for statistical analysis. In the above experimental procedure, the determination is limited to the range of 1.25-80 µmol nitrate g-1 fresh weight. If a result exceeds this limit, the extract should be diluted appropriately using ultrapure water, depending on the nitrate concentration of the sample.
Recipes
- Reaction reagent 1
0.05% (w/v) salicylic acid in sulfuric acid
Prepare freshly each day and avoid exposure to light - Reaction reagent 2
8% (w/v) NaOH in ultrapure water
Store at 4 °C in a polypropylene screw-cap tube with the lid tightly closed
Materials and Reagents
- 0.1-10 µl pipette tips (NIPPON Genetics, catalog number: 30470 )
- 1-200 µl pipette tips (NIPPON Genetics, catalog number: 30430 )
- 100-1,000 µl pipette tips (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 111-N-Q )
- 2.0 ml safe-lock tube (Eppendorf, catalog number: 0107-23-31-33 )
- 5 mm zirconia beads (BMS, catalog number: ZZ50-0001 )
- 1.5 ml microtube (WATSON, catalog number: 131-5155C )
- Assay plate (Iwaki, catalog number: 3881-096 )
- Gloves and eye protection
- Arabidopsis thaliana ecotype Col-0
- Liquid N2
- 1 mol/L hydrochloric acid (Nacalai Tesque, catalog number: 37314-15 )
- 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) (Dojindo, catalog number: 342-01375 )
- Ethylenediamine-N,N,N’,N’-tetraacetic acid (EDTA), disodium salt, dehydrate (Dojindo, catalog number: 345-01865 )
- L-Cysteine (Sigma-Aldrich, catalog number: 168149 )
- Sulfanilamide (Wako Pure Chemical Industries, catalog number: 191-04502 )
- N-1-Naphthylethylenediamine dihydrochloride (Wako Pure Chemical Industries, catalog number: 147-04141 )
- Ultrapure water (Milli-Q, Millipore, 18 MΩ cm)
- Extraction reagent 1 (see Recipes)
- Reaction reagent 3 (see Recipes)
- Reaction reagent 4 (see Recipes)
Equipment
- Pipettes (Nichiryo, model: Nichipet EX II )
- Sample disruptor with beads (QIAGEN, model: TissueLyser II )
Note: Mortar and pestle are practicable. - Refrigerated centrifuge (TOMY DIGITAL BIOLOGY, model: MX-300 )
- Vortex mixer (Scientific Industries, catalog number: SI-0236 )
- Multimode plate reader (PerkinElmer, model: EnSpire® 2300 )
Note: Versatile spectrophotometers are practicable. - Spectrophotometer (Shimadzu, model: UV-1650PC )
Procedure
- Extraction of nitrite from A. thaliana shoots or roots
- Excise and weigh fresh shoots or roots (approximately 50-100 mg fresh weight), put them into 2 ml safe-lock tubes, and freeze them in liquid N2.
Note: We have not checked whether the frozen samples are storable or not. We recommend that the samples should be used for extraction and measurement of nitrite as soon as possible. - Crush frozen samples into a fine powder with a sample disruptor using 5 mm zirconia beads (one bead per tube) or with a mortar and pestle under liquid N2 conditions.
- Add 5 vols. of extraction reagent 1 (see Recipes), vortex gently until the solution dissolves and becomes homogeneous, and keep the extracts on ice.
Note: The volume of extraction buffer added is determined based on the assumption that the pellet derived from 1 mg fresh weight has a volume of about 1 µl. - Centrifuge the extracts at 20,400 x g for 10 min at 4 °C. Use the supernatants for nitrite determination.
Note: The supernatants can be used for measurements of nitrate reductase activity (Konishi and Yanagisawa, 2011).
- Excise and weigh fresh shoots or roots (approximately 50-100 mg fresh weight), put them into 2 ml safe-lock tubes, and freeze them in liquid N2.
- Determination of nitrite from A. thaliana shoots or roots
- Mix 150 µl of the supernatant with 150 µl of reaction reagent 3 (see Recipes) in a 1.5 ml microtube to determine the apparent nitrite concentration.
- Mix 150 µl of the supernatant with 150 µl of 1 mol/L hydrochloric acid instead of reaction reagent 3 to determine the nonspecific background concentration.
- Centrifuge the mixture at 20,400 x g for 10 min at 4 °C to remove precipitates.
- Mix 200 µl of the supernatant with 100 µl of reaction reagent 4 (see Recipes).
Note: Supernatant:reagent 3:reagent 4 = 1:1:1 (v/v/v). If a larger volume is needed to measure the absorbance, this mixture can be scaled up. - Incubate at room temperature for 15 min.
- Measure the absorbance at 540 nm using a spectrophotometer.
- Mix 150 µl of the supernatant with 150 µl of reaction reagent 3 (see Recipes) in a 1.5 ml microtube to determine the apparent nitrite concentration.
- Preparation of nitrite standard curve
- Prepare a nitrite dilution series using potassium nitrite (0, 5, 10, 20, and 40 µM) in the extraction reagent 1 (freshly prepared).
Note: In most plants under normal growth conditions (except for those under anoxia/hypoxia or in which nitrite reduction is suppressed), little nitrite accumulates in the tissues. The range of the standard curve should be adjusted depending on the type of sample. - Mix standard solutions with equal vols. of reaction reagent 3 and reaction reagent 4 in 1.5 ml microtubes.
- Incubate at room temperature for 15 min.
- Measure the absorbance at 540 nm using the spectrophotometer (Figure 3). A picture of a representative nitrite dilution series is shown in Figure 4.
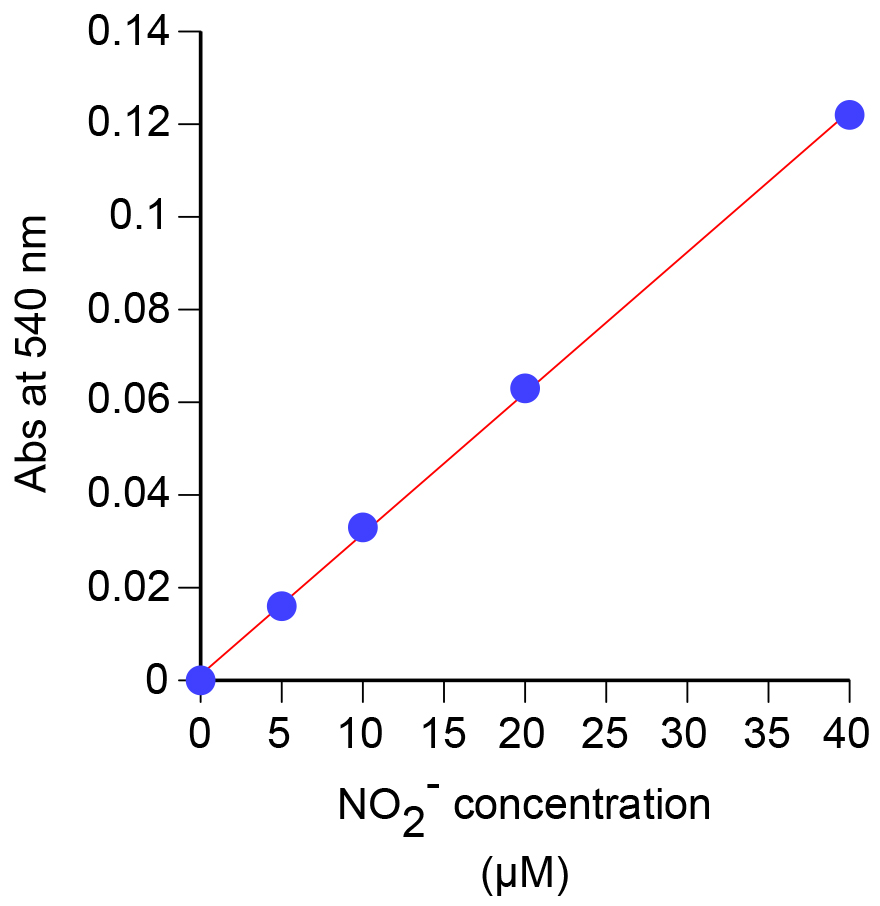
Figure 3. Standard curve generated using the absorbance at 540 nm for known concentrations of nitrite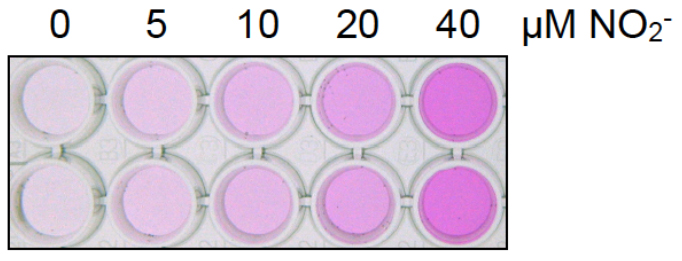
Figure 4. Picture of a representative nitrite dilution series
- Prepare a nitrite dilution series using potassium nitrite (0, 5, 10, 20, and 40 µM) in the extraction reagent 1 (freshly prepared).
Data analysis
- A typical nitrite standard curve is shown in Figure 3. From the curve, the nitrite concentration (µM) can be determined using the formula: (Abs540 - intercept)/slope.
- Calculate the apparent nitrite concentration (µM) of the supernatant using the standard curve.
- Calculate the nonspecific background concentration (µM) of the supernatant using the standard curve.
- Calculate the true nitrite concentration (µM) of supernatant by subtracting the nonspecific value from the apparent value.
- Determine the nitrite content of the sample (nmol g-1 fresh weight) using the formula: [true nitrate concentration (µM)] x [extracted volume (ml)]/[fresh weight (g)].
Notes
Nitrite solutions are not very stable. Extraction and determination should be carried out as soon as possible. Hydrochloric acid is very dangerous and harmful to humans and the environment. Wear gloves and eye protection, and discard waste liquid properly. Please make sure that RISK assessments are carried out before conducting experiments. At least two technical replicates should be obtained for each sample and standard. More than five biological replicates are desirable to obtain reliable data for statistical analysis. In the above experimental procedure, the determination is limited to the range of 25-200 nmol nitrite g-1 fresh weight. If a result exceeds this limit, the extract should be diluted appropriately using extraction reagent 1, depending on the nitrite concentration of the sample. Nitrite is accumulated in tissues where nitrite reduction is inhibited under a limited supply of reducing equivalents (Hachiya et al., 2016).
Recipes
- Extraction reagent 1
50 mM HEPES-KOH (pH 7.6)
1 mM EDTA
7 mM cysteine
Note: Add cysteine just before use. Store solution without cysteine at 4 °C after autoclaving. - Reaction reagent 3
1% (w/v) sulfanilamide in 1 mol/L hydrochloric acid
Prepare freshly each day - Reaction reagent 4
0.02% (w/v) N-1-naphthylethylenediamine dihydrochloride in ultrapure water
Prepare freshly each day
Materials and Reagents
- 0.1-10 µl pipette tips (NIPPON Genetics, catalog number: 30470 )
- 1-200 µl pipette tips (Nippon Genetics, catalog number: 30430 )
- 100-1000 µl pipette tips (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 111-N-Q )
- 2.0 ml safe-lock tube (Eppendorf, catalog number: 0107-23-31-33 )
- 5 mm zirconia beads (BMS, catalog number: ZZ50-0001 )
- 1.5 ml microtube (WATSON, catalog number: 131-5155C )
- Gloves and eye protection
- Assay plate (Iwaki, catalog number: 3881-096 )
- Arabidopsis thaliana ecotype Col-0
- Ultrapure water (Milli-Q, Millipore, 18 MΩ cm)
- Liquid N2
- Chloroform (Wako Pure Chemical Industries, catalog number: 038-02606 )
- Acid-washed activated charcoal (Wako Pure Chemical Industries, catalog number: 035-18081 )
- 1 mol/L hydrochloric acid (Nacalai Tesque, catalog number: 37314-15 )
- Ammonia test kit (Ammonia Test Wako, Wako Pure Chemical Industries, catalog number: 277-14401 )
Notes: - This inexpensive kit ensures reliable measurement of ammonium based on modifications of the Okuda-Fujii method (Okuda and Fujii, 1965).
- The ammonia test kit is ready-to-use and can be stored at 4 °C for one and a half years. Instead of using the kit, it is possible to prepare the reaction reagents using commercial reagents based on the methods of Bräutigam et al. (2007).
- Extraction reagent 2 (see Recipes)
Equipment
- Pipettes (Nichiryo, model: Nichipet EX II )
- Sample disruptor with beads (QIAGEN, model: TissueLyser II )
Note: Mortar and pestle are practicable. - Refrigerated centrifuge (TOMY DIGITAL BIOLOGY, model: MX-300 )
- Heat block (TAITEC, model: DTU-1B )
- Vortex mixer (Scientific Industries, catalog number: SI-0236 )
- Microtube mixer (TOMY DIGITAL BIOLOGY, model: MT-360 )
- Multimode plate reader (PerkinElmer, model: EnSpire® 2300 )
Note: Versatile spectrophotometers are practicable. - Spectrophotometer (Shimadzu, model: UV-1650PC )
Procedure
- Extraction of ammonium from A. thaliana shoots or roots
- Excise and weigh fresh shoots or roots (approximately 10-100 mg fresh weight), put them into 2 ml safe-lock tubes, and freeze them in liquid N2.
Note: Samples can be stored at -80 °C before use. - Crush frozen samples into a fine powder with a sample disruptor using 5 mm zirconia beads (one bead per tube) or with a mortar and pestle under liquid N2 conditions.
- Add 1 ml of extraction reagent 2 (see Recipes), and vortex gently until the solution dissolves and becomes homogeneous.
- Add 500 µl of chloroform.
- Vortex the mixture gently at 4 °C for 15 min.
- Centrifuge the extracts at 12,000 x g for 10 min at 8 °C.
- Transfer the aqueous phase into a 1.5 ml microtube containing approximately 50 mg of acid-washed activated charcoal and then vortex the mixture.
- Centrifuge the extracts at 20,400 x g for 5 min at 8 °C. Use the supernatants for ammonium determination.
Note: A series of purification steps eliminates interfering compounds for ammonium determination.
- Excise and weigh fresh shoots or roots (approximately 10-100 mg fresh weight), put them into 2 ml safe-lock tubes, and freeze them in liquid N2.
- Determination of ammonium from A. thaliana shoots or roots
- Mix 200 µl of the supernatant with 800 µl of the deproteinization reagent in the ammonia test kit in a 1.5 ml microtube and vortex.
Note: The deproteinization reagent contains sodium tungstate and phosphate salt. - Centrifuge the mixture at 2,300 x g for 5 min at 4 °C.
- Mix 200 µl of the supernatant with 200 µl of reagent A in the ammonia test kit and vortex.
Note: Reagent A contains sodium nitroprusside and phenol. - Add 100 µl of reagent B in the ammonia test kit and vortex.
Note: Reagent B contains potassium hydroxide. - Add 200 µl of reagent C in the ammonia test kit and vortex.
Note: Reagent C contains sodium hypochlorite and potassium carbonate. - Incubate at 37 °C for 20 min.
- Measure the absorbance at 630 nm using a spectrophotometer.
Note: If the ammonium concentration exceeds the range of the standard dilution series, dilute the supernatant with the extraction reagent 2. In general, ammonium accumulates at higher levels in plant tissues grown under ammonium-containing conditions.
- Mix 200 µl of the supernatant with 800 µl of the deproteinization reagent in the ammonia test kit in a 1.5 ml microtube and vortex.
- Preparation of ammonium standard curve
- Prepare an ammonium dilution series using ammonium chloride (0, 12.5, 25, 50, 100, 200, and 400 µM) in the extraction reagent 2 (freshly prepared).
Note: Ammonia Test Wako includes solutions for the preparation of the dilution series. However, their background composition resembles deproteinized blood because this kit is designed to measure ammonium in blood. Thus, the dilution series should be prepared with the extraction reagent 3. - Mix 200 µl of the standard solution with 800 µl of the deproteinization reagent in the ammonia test kit in a 1.5 ml microtube and vortex.
Note: The deproteinization reagent contains sodium tungstate and phosphate salt. - Centrifuge the mixture at 800 x g for 5 min at 4 °C.
- Mix 200 µl of the supernatant with 200 µl of reagent A in the ammonia test kit and vortex.
- Add 100 µl of reagent B in the ammonia test kit and vortex.
- Add 200 µl of reagent C in the ammonia test kit and vortex.
- Incubate at 37 °C for 20 min.
- Measure the absorbance at 630 nm using the spectrophotometer (Figure 5). A picture of a representative ammonium dilution series is shown in Figure 6.
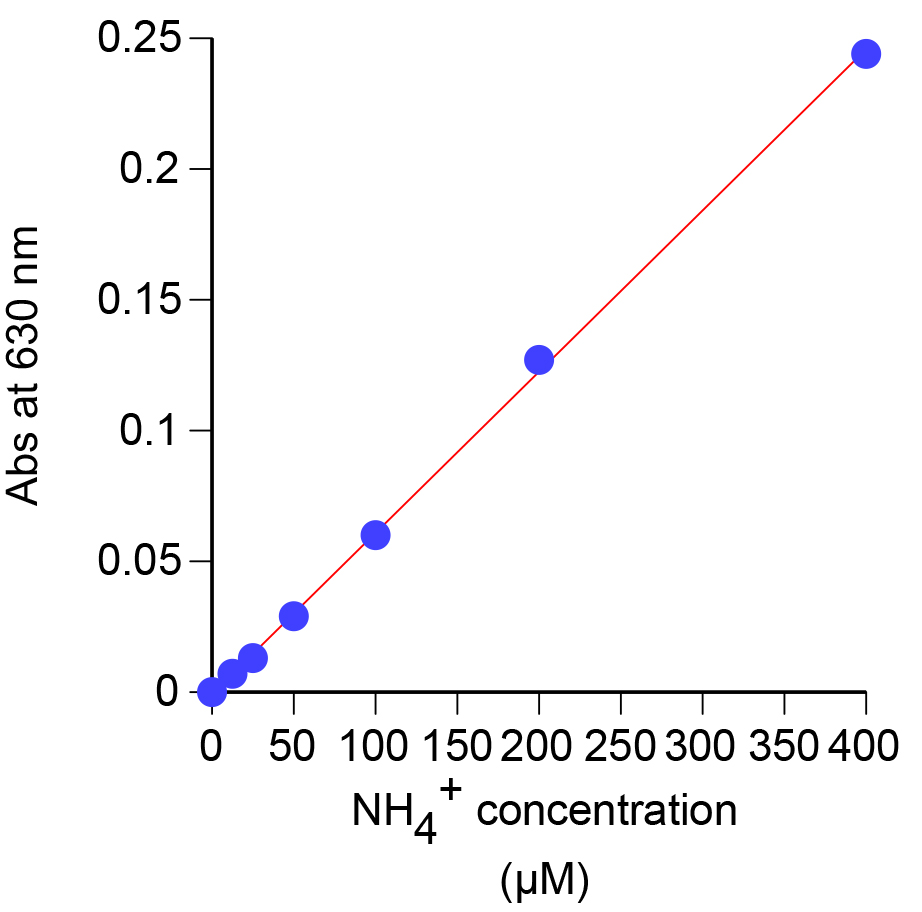
Figure 5. Standard curve generated using the absorbance at 630 nm for known concentrations of ammonium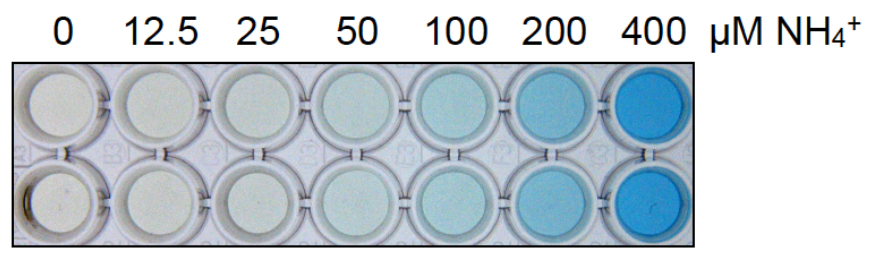
Figure 6. Picture of a representative ammonium dilution series
- Prepare an ammonium dilution series using ammonium chloride (0, 12.5, 25, 50, 100, 200, and 400 µM) in the extraction reagent 2 (freshly prepared).
Data analysis
- A typical ammonium standard curve is shown in Figure 5. From the curve, the ammonium concentration (µM) in the supernatant can be determined using the formula: (Abs630 - intercept)/slope.
- Determine the ammonium content of the sample (µmol g-1 fresh weight) using the formula: [ammonium concentration (µM)] x [extracted volume (ml)]/[fresh weight (g)]/1,000.
Notes
Chloroform should be used in a fume hood. Hydrochloric acid is very dangerous and harmful to humans and the environment. Wear gloves and eye protection, and discard waste liquid properly. Acid-washed activated charcoal should be used carefully in a fume hood to ensure that dust is not scattered. Please make sure that RISK assessments are carried out before conducting experiments. At least two technical replicates should be obtained for each sample and standard. More than five biological replicates are desirable to obtain reliable data for statistical analysis. In the above experimental procedure, a linear standard curve is not always achieved below 12.5 µM or above 400 µM. If a result exceeds this limit, the extract should be diluted appropriately using extraction reagent 2, depending on the ammonium concentration of the sample. High ammonium contents are accumulated in plants grown under ammonium at millimolar concentrations, but little ammonium is accumulated under nitrate conditions (Hachiya et al., 2012).
Recipes
- Extraction reagent 2
0.1 mol/L hydrochloric acid
Acknowledgments
The present procedures are derived from the work of Cataldo et al. (1975), Rockel et al. (2002), Bräutigam et al. (2007), Konishi and Yanagisawa (2011), and Hachiya et al. (2012 and 2016). This work was supported by Building of Consortia for the Development of Human Resources in Science and Technology and by Core Research for Evolutional Science and Technology, Japan Science and Technology Agency.
References
- Bräutigam, A., Gagneul, D. and Weber, A. P. M. (2007). High-throughput colorimetric method for the parallel assay of glyoxylic acid and ammonium in a single extract. Anal Biochem 362(1): 151-153.
- Cataldo, D. A., Haroon, M., Schrader, L. E. and Youngs, V. L. (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6: 71-80.
- Hachiya, T., Ueda, N., Kitagawa, M., Hanke, G., Suzuki, A., Hase, T. and Sakakibara, H. (2016). Arabidopsis root-type ferredoxin:NADP(H) oxidoreductase 2 is involved in detoxification of nitrite in roots. Plant Cell Physiol 57(11): 2440-2450.
- Hachiya, T., Watanabe, C. K., Fujimoto, M., Ishikawa, T., Takahara, K., Kawai-Yamada, M., Uchimiya, H., Uesono, Y., Terashima, I. and Noguchi, K. (2012). Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant Cell Physiol 53(3): 577-591.
- Konishi, M. and Yanagisawa, S. (2011). The regulatory region controlling the nitrate-responsive expression of a nitrate reductase gene, NIA1, in Arabidopsis. Plant Cell Physiol 52(5): 824-836.
- Okuda, H. and Fujii, S. (1966). Determination of blood ammonia by the spectrophotometric method. Saishin Igaku 21: 622-627.
- Rockel, P., Strube, F., Rockel, A., Wildt, J. and Kaiser, W. M. (2002). Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53(366): 103-110.
- Xu, N., Wang, R., Zhao, L., Zhang, C., Li, Z., Lei, Z., Liu, F., Guan, P., Chu, Z., Crawford, N. M. and Wang, Y. (2016). The Arabidopsis NRG2 protein mediates nitrate signaling and interacts with and regulates key nitrate regulators. Plant Cell 28(2): 485-504.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hachiya, T. and Okamoto, Y. (2017). Simple Spectroscopic Determination of Nitrate, Nitrite, and Ammonium in Arabidopsis thaliana. Bio-protocol 7(10): e2280. DOI: 10.21769/BioProtoc.2280.
Category
Plant Science > Plant metabolism > Nitrogen
Cell Biology > Cell metabolism > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









