- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Electroshock Induced Seizures in Adult C. elegans
Published: Vol 7, Iss 9, May 5, 2017 DOI: 10.21769/BioProtoc.2270 Views: 9059
Reviewed by: Neelanjan BoseSanjib Kumar GuhaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Evaluation of Burkholderia cepacia Complex Bacteria Pathogenicity Using Caenorhabditis elegans
Pietro Tedesco [...] Donatella de Pascale
Oct 20, 2016 7697 Views

Artificial Optogenetic TRN Stimulation of C. elegans
Ithai Rabinowitch [...] Jihong Bai
Oct 20, 2016 8621 Views

Aerotaxis Assay in Caenorhabditis elegans to Study Behavioral Plasticity
Qiaochu Li [...] Karl Emanuel Busch
Aug 20, 2022 2252 Views
Abstract
The nematode Caenorhabditis elegans is a useful model organism for dissecting molecular mechanisms of neurological diseases. While hermaphrodite C. elegans contains only 302 neurons, the conserved homologous neurotransmitters, simpler neuronal circuitry, and fully mapped connectome make it an appealing model system for neurological research. Here we developed an assay to induce an electroconvulsive seizure in C. elegans which can be used as a behavioral method of analyzing potential anti-epileptic therapeutics and novel genes involved in seizure susceptibility. In this assay, worms are suspended in an aqueous solution as current is passed through the liquid. At the onset of the shock, worms will briefly paralyze and twitch, and shortly after regain normal sinusoidal locomotion. The time to locomotor recovery is used as a metric of recovery from a seizure which can be reduced or extended by incorporating drugs that alter neuronal and muscular excitability.
Keywords: EpilepsyBackground
We were interested in using the powerful genetic model, Caenorhabditis elegans, to develop an electroconvulsive seizure assay that can be easily manipulated by pharmacology. Invertebrate models have been used in seizures research for decades (Lee and Wu, 2002) however there were no protocols specifically investigating electroconvulsive seizures in C. elegans. In the past, multiple groups have developed methods of analyzing paralysis in response to chemical proconvulsants such as the GABAA receptor blockers, pentylentetrazol (PTZ) and picrotoxin (PTX), as well as an acetylcholinesterase inhibitor, aldicarb (Williams et al., 2004; Vashlishan et al., 2008). While these methods typically analyze the time to paralysis, our method quantifies the time it takes to recover from an electric shock-induced seizure (Risley et al., 2016).
Materials and Reagents
- 60 x 15 mm Petri dishes (Excel Scientific, catalog number: D-901 )
- Pipette tips (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 0094300120 )
- Vacuum filter (Corning, catalog number: 430320 )
- 2, 20 gauge insulated copper wire, cut to 8 cm segments (Del City, catalog number: 1120101 )
- Plastic tubing, cut into 9 mm segments (Emurdock, catalog number: AAQ04127 )
Note: This product has been discontinued. - 2 x Alligator clip wires (United Scientific Supplies, catalog number: WAG024-PK/6 )
- C. elegans wild type strain N2 (obtained from Caenorhabditis Genetics Center)
- LB broth powder (Fisher Scientific, catalog number: BP1426-500 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014 )
- Agar (Sigma-Aldrich, catalog number: A7002 )
- Peptone (Fisher Scientific, catalog number: BP1420 )
- Calcium chloride solution (CaCl2) (Sigma-Aldrich, catalog number: 21115 )
- Magnesium sulfate solution (MgSO4) (Sigma-Aldrich, catalog number: M3409 )
- Cholesterol (Sigma-Aldrich, catalog number: C8667 )
- Ethanol (Fisher Scientific, catalog number: 04-355-226 )
- Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P3786 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: 795410 )
- LB broth (see Recipes)
- Nematode growth medium (NGM) agar plates (see Recipes)
- KP buffer (see Recipes)
- E. coli strain OP50 (see Recipes)
- M9 solution (see Recipes)
Equipment
- 2 L flask
- Pipettes
- Stir bar
- Dissection stereo microscope (Tritech Research, model: SMT1 )
- Dissecting stereoscope (AmScope, model: SM-1TSX )
- Timer output stimulator (Grass Instruments, model: S44 )
- Stimulator (Grass Instruments, model: SD9 )
- CCD color microscope camera (Hitachi, model: KP-D20BU )
- Television monitor (RCA, model: 19LA30RQ )
- HDD and DVD recorder (Magnavox, catalog number: MDR535H/F7 )
- DVD-Recordable discs (Verbatim, catalog number: 95032 )
- Digital oscilloscope (OWON Technology, catalog number: PDS5022T )
- Ethanol lamp (Carolina, catalog number: 706604 )
- Water bath (50 °C) (Corning, model: Corning® LSETM Digital Water Bath, catalog number:6783)
- Incubators (37 °C) (Thelco, model: 4 )
- Incubator (20 °C) (Cuisinart, catalog number: CWC 1200DZ )
- Timer (VWR, catalog number: 62344-641 )
- A metric ruler (Fisher Scientific, catalog number: 09-016 )
- Infrared Temperature Gun (J-1 Trading Wholesale, Nubee, catalog number: NUB8380 )
Software
- Open source image processing program, we use VLC media player (version 2.2.4)
- SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA, USA)
Procedure
- Set up the microscope camera to the TV and digital video recorder as per the camera instructions (Figures 1 and 2A).
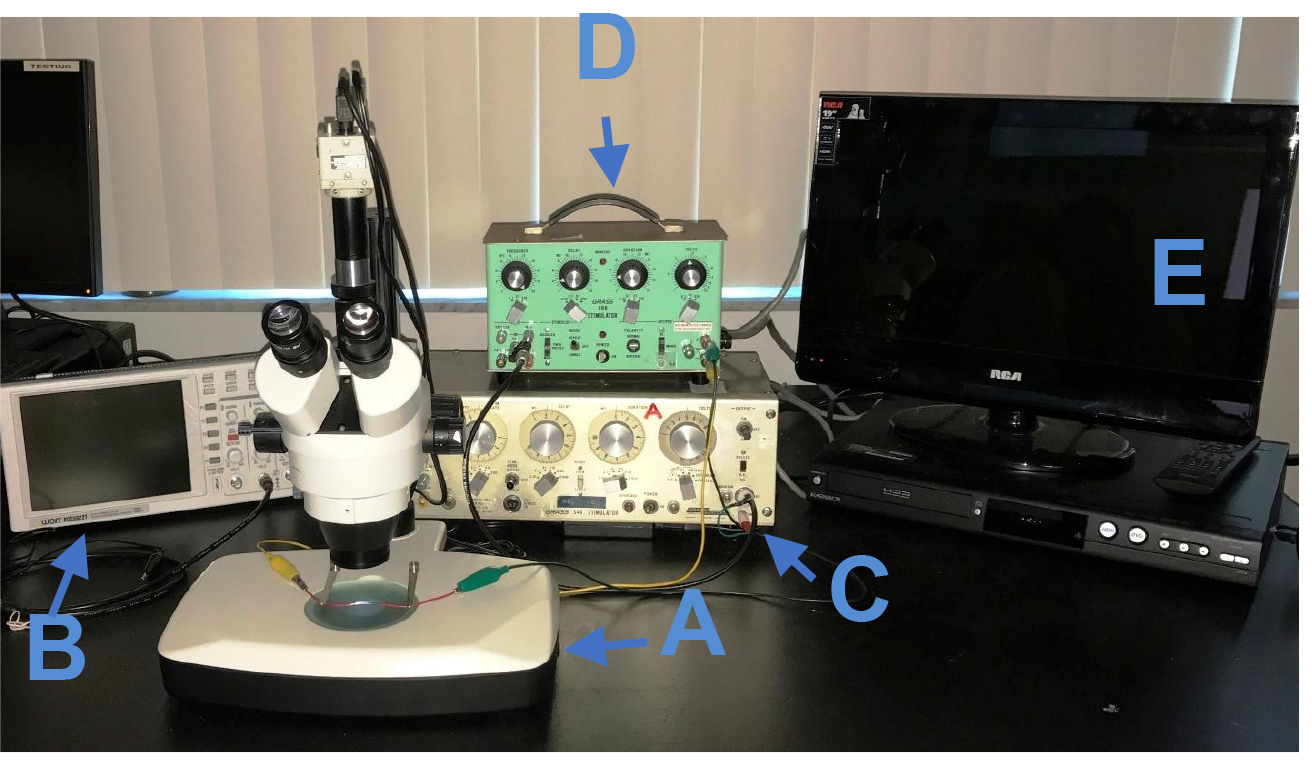
Figure 1. The experimental setup. The experimental setup includes a stereoscope (A), oscilloscope (B), timer output stimulator (S44) (C), stimulator (SD9) (D), and the recording equipment (E).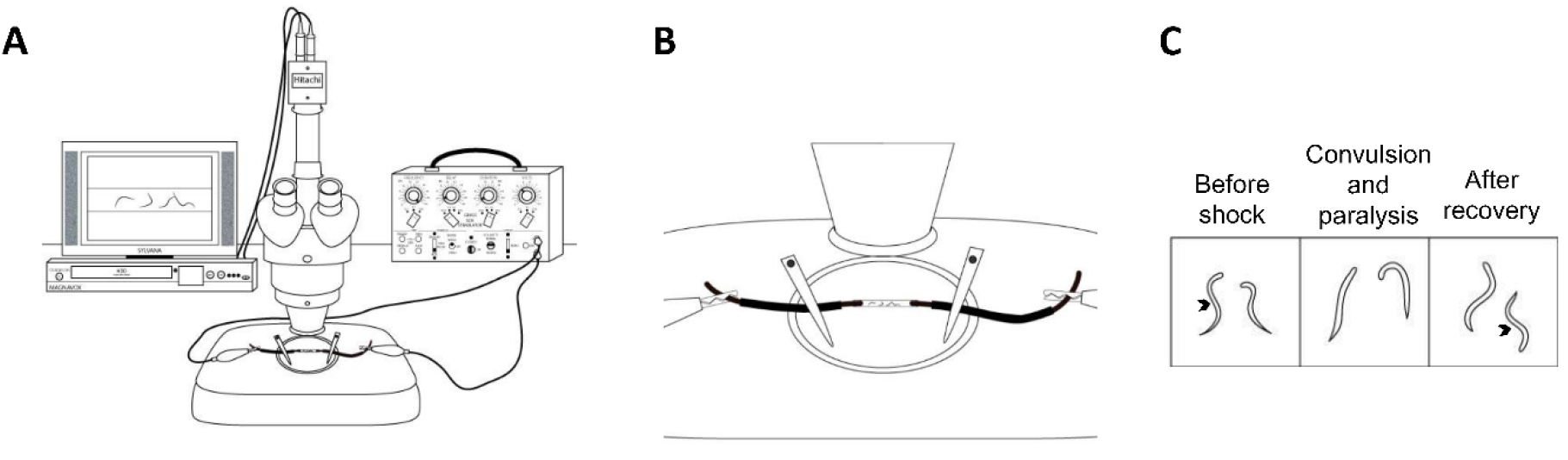
Figure 2. A simplified schematic of the experimental setup and analysis. A. The stereoscope (center), the recording equipment (left), and a stimulator (right) represent a simplified schematic of a basic experimental setup. To control duration, our setup includes an additional stimulator (S44); however, timing can be controlled by many different methods. B. A close up schematic of the experimental tube plugged on either end with copper wires; C. This schematic represents the normal, sinusoidal body position (arrow) of a worm before the shock. During and after the shock, the worms generally exhibit convulsions and paralysis, and then typically resume normal sinusoidal locomotion. This figure was adapted from Risley et al., 2016. - Connect the output on the S44 stimulator via a cable with a banana plug splitter and connect it to the ground and mod banana plug inputs on the SD9 stimulator. The goal here is to connect the S44 to the SD9 stimulator so that the S44 can be used to modulate duration.
Note: Be sure the polarity of the banana plugs is consistent. - Set the S44 to at least 5 V and desired stimulus duration; we use 3 sec.
- Clip one of the alligator clips onto the positive (+) output on the SD9 and the other onto the negative (-) output. These alligator clip wires will connect the SD9 stimulator to the experimental tube (Figures 3 and 2B).
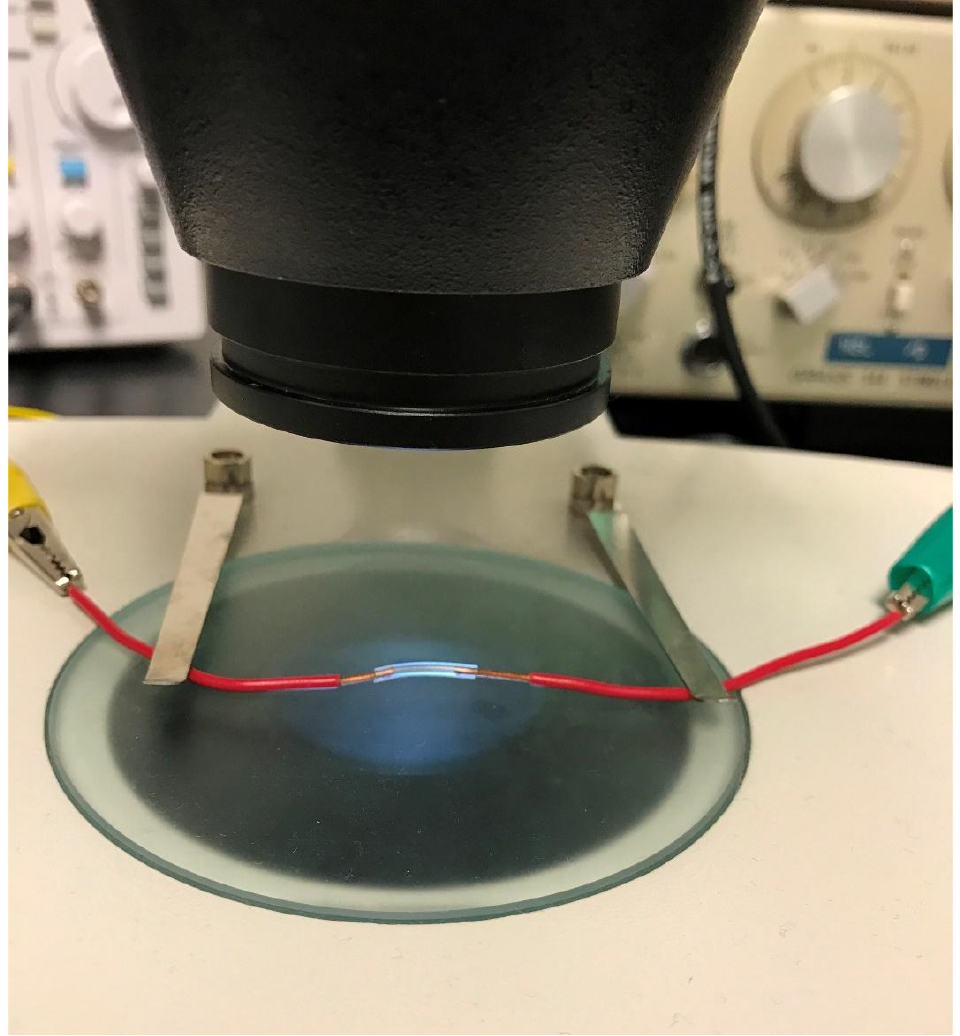
Figure 3. Close-up image of the experimental tube. The tube is plugged on either end with the insulated copper wires that are attached to the stimulator. The distance between the copper wire should be exactly 10 mm. - Next clip each of the copper wires to the open end of each alligator clip. The open end of each copper wire will later be plugged directly into experimental tube when the experiment is ready to begin. Set up the microscope camera to the TV as per the camera instructions (Figures 1 and 2A).
Note: It is very important to set up the experimental tubes consistently to obtain a consistent. - 1-day old adult Caenorhabditis elegans are used for the experiments. 24-h before experimentation, approximately 30 L4 stage worms per genotype are transferred to fresh NGM plates seeded with OP50 E. coli. The worms are placed in a 20 °C incubator overnight; and will mature into 1-day old adults in approximately 18 h. During experimentation the following day, the worm plates are left on the lab bench at room temperature (23 °C).
- Typically, the drug solutions are prepared on the morning of experimentation. The drugs are dissolved directly into a vial of M9 solution with a total volume of 3-5 ml, depending on the number of experimental trials. Sham controls are also prepared at this time. The prepared reagents can be stored, at 4 °C or based on the specifications of the drug, for experiments performed on consecutive days.
Note: Water-soluble drugs were dissolved directly into M9 (Risley et al., 2016); however, a DMSO concentration curve was conducted in Risley et al. (2016) and it was reported that DMSO concentrations up to 0.5% had no significant effect on wild-type locomotor recovery after electric shock. - Next, load a blank DVD+R into the HDD/DVD recorder and turn on the TV screen. Also, verify that the settings are correct on the timer output stimulator and stimulator using the oscilloscope. We typically set the electroshock parameters to 47 V, 3 sec duration. This voltage was selected based off a voltage-response curve. The maximum voltage in which wild-type worms recovered was about 60 V and they recovered, on average, in 85 sec. 47 V was selected as worms recover in approximately one half the time of maximum limit for recovery (Figure 4).
Note: The timer output stimulator (S44) is used only as a trigger for the SD9 stimulator, which sends current to the worms. The duration, typically 3 sec, is set on the S44 and used as a trigger to regulate the duration. There are many other ways to control for the duration including using a digital trigger via a computer or an Arduino.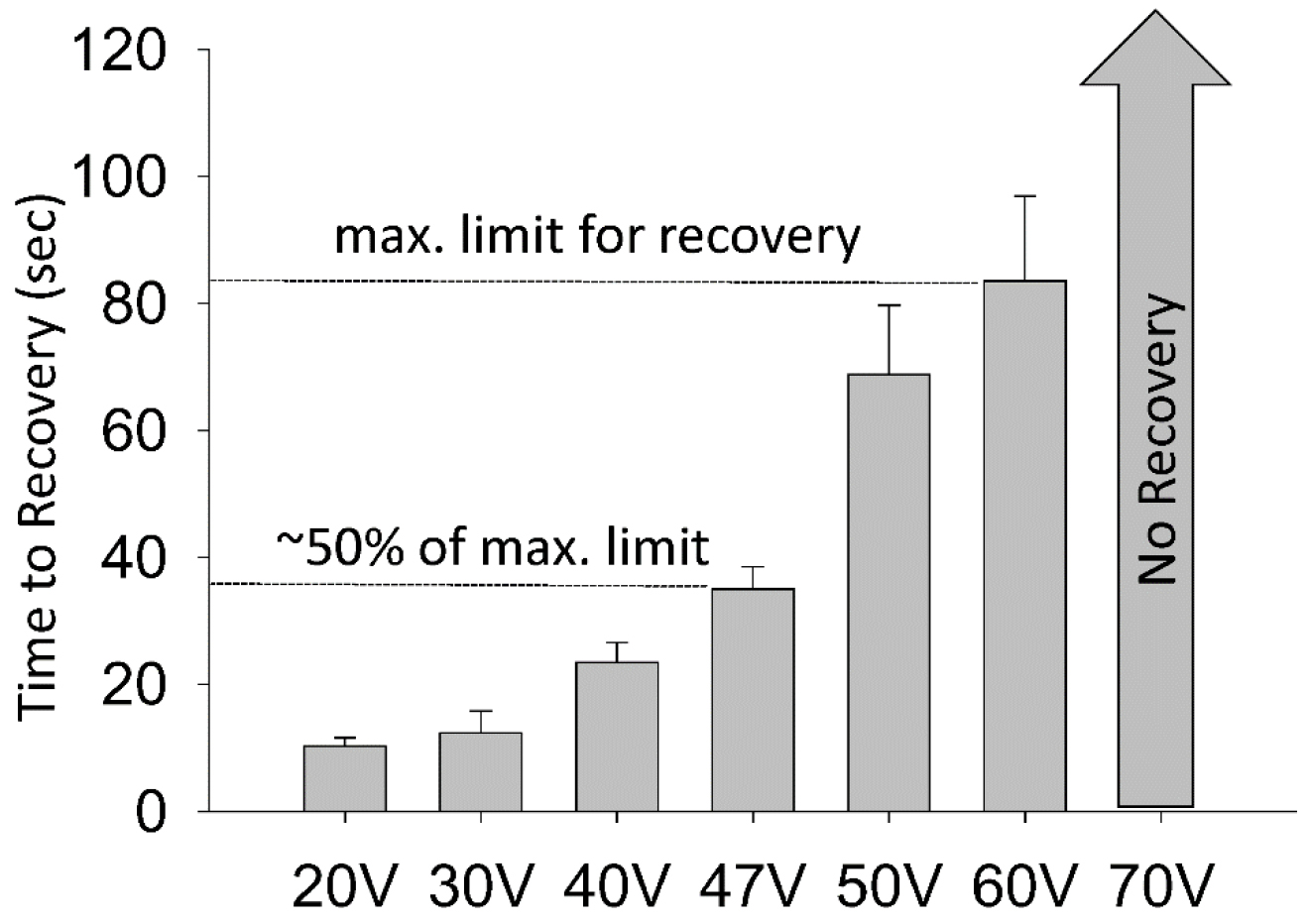
Figure 4. A voltage response curve demonstrates locomotor recovery times in wild-type worms. Wild-type worms did not recover at voltages ≥ 70 V. The maximum tested limit for recovery was 60 V where worms recovered in approximately 80 seconds. 47 V was the voltage in which recovery time was 50% of the maximum limit. This figure was adapted from Risley et al., 2016. - Load approximately 20 µl of control solution or drug solution. Immediately following, load 6-8 worms into the tube using a platinum pick and set a timer for 30 min. 30 sec before the timer expires, gently place the tube under the microscope/camera setup with the light base turned on, and plug each copper wire into the two ends of the tube; the wires should be 10 mm apart (Figures 3 and 2B). Verify the distance between the copper wires is precise (a ruler will suffice) and initiate the electroshock. Allow the experiment to record for 10 min. After the 10 min recording, the tube and its contents are discarded.
Note: Generally, a tube is loaded every 10 min. Each tube is shocked and recorded for 10 min before it is discarded and another tube is immediately prepared. Also, OP50 transferred from the platinum pick to the tube should be minimized as it could impede the view of the worms.
Data analysis
- The analysis is manually scored using a video player. Each worm is scored individually in each tube. An example screenshot of a raw video file can be seen in Figure 5 and a schematic representation in Figure 2C. The time of the shock onset (depicted by the appearance of bubbles) is recorded in a notebook and subsequent recovery times of each worm are recorded.
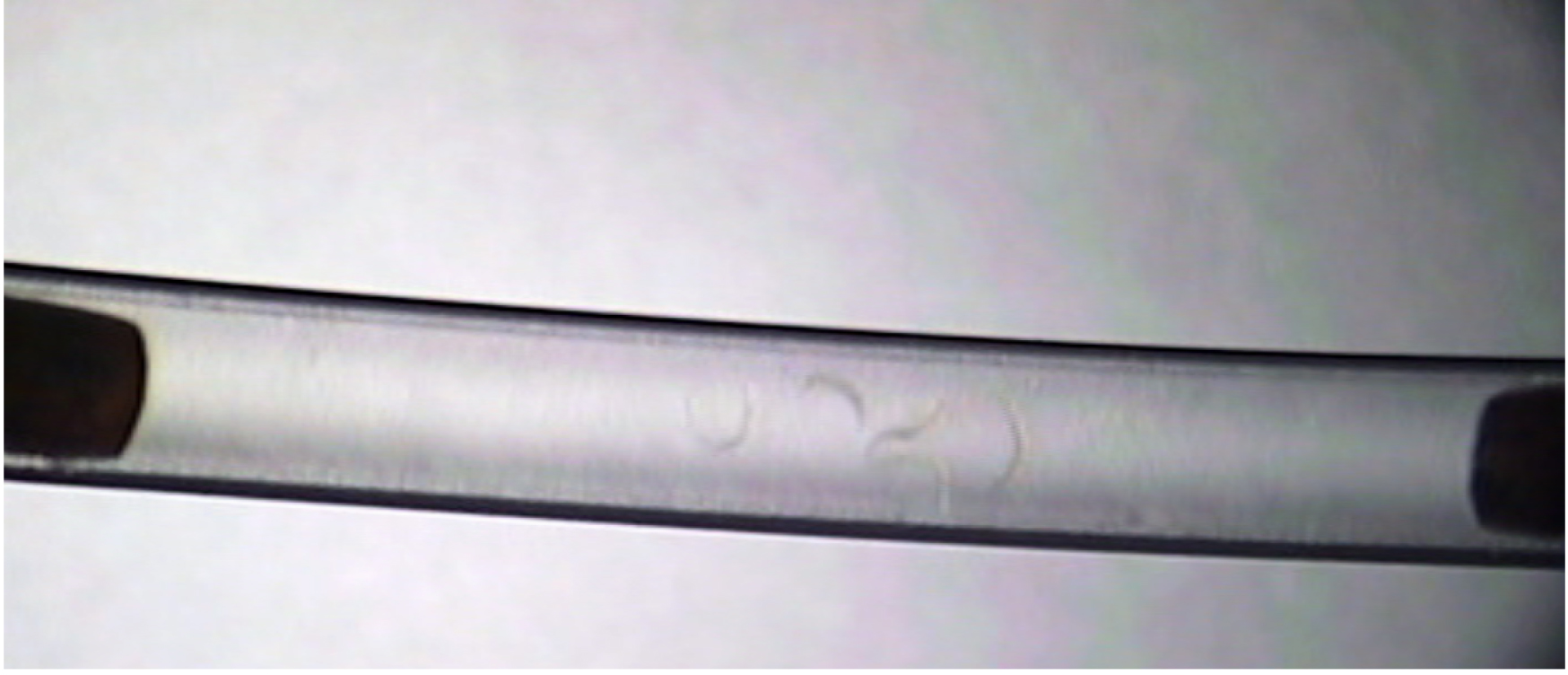
Figure 5. Example screenshot of recorded raw data. The image is a screenshot from a raw data video file of an experiment. There are five worms centered inside the middle of the tube. The tube is plugged by the copper wire on either end. This is an example of the video file that is analyzed by the researcher. - Recovery is defined as a worm regaining its ability to move in a sinusoidal movement, similar to the locomotion it exhibited prior to the shock. Partial recovery of only head or tail is not considered recovery. (Video 1)
- Recovery time of each worm was considered n = 1 and the total number of experimental tubes was N = 1. For all experiments, a full data set is considered n ≥ 10 and N ≥ 6.
- Data is analyzed in SigmaPlot using One-Way ANOVA followed by a post-hoc Multiple Comparison’s test or a Student’s t-test; however, appropriate statistical analysis should be determined by the researcher. Data was typically represented by a bar graph ± SEM. An example of the data bar graphs can be seen in the voltage-response curve in Figure 4.Video 1. Example video of the experimental setup and analysis. This video shows another visual of the experimental setup, followed by a raw data video file. The video is annotated with notes describing when each worm is considered recovered. This figure was adapted from Risley et al., 2016.
Notes
- Due to electrolysis during the stimulus, bubbles will form on the edge of the tube. Worms that are covered by the bubbles are excluded from analysis. We have used an infrared temperature gun to record temperature fluctuations over 3 sec. The temperature fluctuations are acute and minimal, 1 ± 0.5 °C, however temperature should be recorded to be sure increased temperature will not compound the effect of the electroshock. If the shock is administered for more than 3 sec, care should be taken that the temperature does not increase substantially as this could confound the results.
- The speed of the sinusoidal locomotion was not taken into consideration during analysis.
- Worms that did not recover were excluded from analysis.
Recipes
- LB broth
Add 25 g LB broth powder and 1 L dH2O to a 2 L flask
Autoclave for 30 min - Nematode growth medium (NGM) agar plates
- Add 3 g NaCl, 17 g agar, 2.5 g peptone, and 975 ml dH2O to a 2 L flask
- Autoclave for 30 min
- Let cool to 50 °C in the water bath
- Add 1 ml 1 M CaCl2, 1 ml 1 M MgSO4, 5 mg cholesterol dissolved in 1 ml 95% ethanol, 25 ml KP buffer to the agar solution, mixing well after each addition
- Pipette 10 ml into each 60 x 15 mm Petri dish
- Cover and store at 4 °C
- When needed, seed plates with 50 µl of OP50 E. coli and incubate overnight at 37 °C
- KP buffer
Add 2.46 g KH2PO4 and 1.205 g K2HPO4, to 25 ml diH2O with a stir bar
Adjust pH to 6.0 and vacuum filter - OP50 E. coli
Suspend 1 colony of OP50 E.coli from a stock plate in LB broth
Incubate overnight at 37 °C with the lid loosely on
Store at 4 °C - M9 solution
Add 3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4 to a 2 L flask and fill with diH2O to 1 L
Autoclave for 15 min and store at room temperature
Acknowledgments
We have published this protocol in (Risley et al., 2016; Opperman et al., 2017). Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
- Lee, J. and Wu, C. F. (2002). Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J Neurosci 22(24): 11065-11079.
- Opperman, K. J., Mulcahy, B., Giles, A. C., Risley, M. G., Birnbaum, R. L., Tulgren, E. D., Dawson-Scully, K., Zhen, M. and Grill, B. (2017). The HECT family ubiquitin ligase EEL-1 regulates neuronal function and development. Cell Rep 19(4): 822-835.
- Risley, M. G., Kelly, S. P., Jia, K., Grill, B. and Dawson-Scully, K. (2016). Modulating behavior in C. elegans using electroshock and antiepileptic drugs. PLoS One 11(9): e0163786.
- Vashlishan, A. B., Madison, J. M., Dybbs, M., Bai, J., Sieburth, D., Ch'ng, Q., Tavazoie, M. and Kaplan, J. M. (2008). An RNAi screen identifies genes that regulate GABA synapses. Neuron 58(3): 346-361.
- Williams, S. N., Locke, C. J., Braden, A. L., Caldwell, K. A. and Caldwell, G. A. (2004). Epileptic-like convulsions associated with LIS-1 in the cytoskeletal control of neurotransmitter signaling in Caenorhabditis elegans. Hum Mol Genet 13(18): 2043-2059.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Risley, M. G., Kelly, S. P. and Dawson-Scully, K. (2017). Electroshock Induced Seizures in Adult C. elegans. Bio-protocol 7(9): e2270. DOI: 10.21769/BioProtoc.2270.
- Risley, M. G., Kelly, S. P., Jia, K., Grill, B. and Dawson-Scully, K. (2016). Modulating behavior in C. elegans using electroshock and antiepileptic drugs. PLoS One 11(9): e0163786.
Category
Neuroscience > Behavioral neuroscience > Animal model > Other
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









