- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Haustorium Induction Assay of the Parasitic Plant Phtheirospermum japonicum
Published: Vol 7, Iss 9, May 5, 2017 DOI: 10.21769/BioProtoc.2260 Views: 10298
Reviewed by: Marisa RosaXinyan ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Maize Seedlings Colonization with Serendipita indica and Its Colonization Efficiency Analysis
Om Prakash Narayan [...] Atul Kumar Johri
Oct 20, 2023 2276 Views

ClearDepth Method for Evaluations of Root Depth in Soil-Filled Pots
Michel Ruiz Rosquete [...] Wolfgang Busch
Aug 20, 2025 2129 Views
Abstract
Phtheirospermum japonicum is a facultative root parasitic plant in the Orobanchaceae family used as a model parasitic plant. Facultative root parasites form an invasive organ called haustorium on the lateral parts of their roots. To functionally characterize parasitic abilities, quantification of haustorium numbers is required. However, this task is quite laborious and time consuming. Here we describe an efficient protocol to induce haustorium in vitro by haustorium-inducing chemicals and host root exudate treatments in P. japonicum.
Keywords: ParasitismBackground
Parasitic plants have evolved to obtain nutrients from other plants. Some of parasitic plants cause significant damage to agriculture by infecting commercial crops (Spallek et al., 2013). Obligate parasitic plants require hosts to complete their lifecycle, while facultative parasitic plants can survive without hosts as autotrophic organisms but shift to heterotrophic by infection if host plants are nearby (Westwood et al., 2010). The common characteristic of all parasitic plants is a specialized organ called haustorium, which connects parasite with host by establishing vascular bridges (Saucet and Shirasu, 2016; Yoshida et al., 2016). Obligate root parasites form terminal haustoria that are derived from enlarged root tips, while facultative root parasites form lateral haustoria, which develop at the lateral side of the parasite roots without affecting the root meristem. Therefore, several lateral haustoria can form in a root. The early stage of haustorium development is characterized by enlarged root tissues caused by a combination of cell expansion and cell division. Several host-derived substances that are able to induce haustorium formation in vitro were previously identified. Such substances are called haustorium-inducing factors (HIF). Among them, the most active HIF is DMBQ (2,6-Dimethoxy-1,4-benzoquinone), initially isolated from sorghum root extracts (Chang and Lynn, 1986). Phtheirospermum japonicum, a facultative parasitic plant in the Orobanchaceae, is an ideal model to study the molecular mechanisms of the parasitism, because of its short life cycle, small size, and simple genetics as a selfing plant (Ishida et al., 2011; Cui et al., 2016). In addition, genetic manipulation of P. japonicum is now feasible (Ishida et al., 2011) and its large-scale transcriptome information is also available (Ishida et al., 2016). Here we report an efficient in vitro method for haustorium induction to investigate functionality of haustorium-related genes in P. japonicum. This method presents a step-by-step protocol for haustorium induction in vitro by DMBQ treatment or by contact with host exudates. This technique is useful to understand the genetic factors that trigger haustorium formation in parasitic plants.
Materials and Reagents
- Falcon 50 ml conical centrifuge tube (e.g., Corning, Falcon®, catalog number: 352070 )
- Filter paper, No. 2, Ø9 cm (Advantec, catalog number: 00021090 )
- Sterilized plastic plate dish with diameter of 100 mm (e.g., BioLite φ100 TC Dish, Fisher Scientific, catalog number: 12-556-002 )
Manufacturer: Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 130182 . - Eppendorf® microcentrifuge tube (1.5 ml) (e.g., Fisher Scientific, catalog number: 05-408-129 )
- Surgical tape (or Parafilm) (e.g., 3M, catalog number: 1530-1 )
- Kitchen aluminium foil
- Sterilized square plastic plate dish 140 x 100 x 14.5 mm (Eiken Chemical, catalog number: AW2000 703077 )
- Microscope slides (e.g., Fisher Scientific, catalog number: 12-549-3 )
- Microscope coverslips L x W x D: 22 x 70 x 1.0 mm (e.g., Fisher Scientific, catalog number: 10-016-24 )
- Gloves
- Rice seeds (Oryza sativa japonica variety Nipponbare)
- Phtheirospermum japonicum seeds
- Commercial hypochlorite solution (Kao Japan) (approx. 6% sodium hypochloride)
- Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 484016 )
- Tween 20 (Sigma-Aldrich, catalog number: P9416 )
- Water (Milli-Q grade)
- Propidium iodide (Sigma-Aldrich, catalog number: P4170 )
- Murashige and Skoog salts (Pre-mixed) (Wako Pure Chemical Industries, catalog number: 392-00591 )
- Sucrose (Merck Millipore, catalog number: 107687 )
- Myo-inositol (Sigma-Aldrich, catalog number: I7508 )
- 2,6-dimethoxy-1,4-benzoquinone (DMBQ) (Sigma-Aldrich, catalog number: 428566 )
- Agar (Merck Millipore, catalog number: 101614 )
- Dimethyl sulfoxide (DMSO) (Wako Pure Chemical Industries, catalog number: 041-29351 )
- Chloral hydrate (Sigma-Aldrich, catalog number: V000554 )
- Glycerol (Sigma-Aldrich, catalog number: G5516 )
- GM media (see Recipes)
- DMBQ stock solution (10 mM) (see Recipes)
- Chloral hydrate solution (see Recipes)
Equipment
- Rice husker (Fujiwara Scientific, model: Testing rice husker )
- Tube rotator (e.g., TITEC, model: RT-50 , catalog number: 0000165-000)
- Laminar flow hood (e.g., YAMATO SCIENTIFIC, model: CCV-1300E )
- Plant growth chamber (e.g., NKsystem, model: LPH-411SP )
- Daylight-white fluorescent lamp (NEC LIGHTING, model: FL40SEX-N-HG )
- Microscope (Leica Microsystems, model: TCS-SP5 II )
- Vortex shaker (e.g., Scientific Industries, model: Vortex-Genie2 , catalog number: G560-SI-0246 2)
- Surgical scalpel handle (e.g., Swann-Morton, catalog number: 0933 )
- Surgical scalpel blade number 11 (e.g., Swann-Morton, catalog number: 0303 )
- Stainless steel forceps (e.g., Sigma-Aldrich, catalog number: F4142-1EA )
- Semi-analytical balance (e.g., Shimadzu, model: AUW220D )
- Water bath (e.g., Fisher Scientific, model: Fisher ScientificTM IsotempTM General Purpose Deluxe Water Bath, catalog number: S28124 )
- Light stereo microscope (e.g., Carl Zeiss, model: Stemi-2000 )
- Light microscope (e.g., Olympus, model: BX53-P )
- Autoclave (e.g., Hirayama, model: HG series )
Procedure
- Rice seed germination
- Remove the seed coats from rice seeds by a rice husker.
Note: If the rice husker is not available, it is possible to remove the seed coat one by one by carefully pinching the seed’s awn with forceps or hands and pushing it down. - Place the coat-removed seeds in a 50 ml plastic tube.
Note: Maximum of ~20-30 seeds per tube. - To surface sterilize the rice seeds, immerse them in 50% (v/v) commercial hypochlorite solution (~3% sodium hypochlorite in final solution) with 0.1% (v/v) Tween 20. Shake the seeds using a vortex set at maximum speed for 5 min and place the plastic tube in a tube rotator for 25 min set to 10-20 rpm.
- From this step, the procedures should be done in a sterile laminar flow hood, wash the seeds with sterilized deionized water (Milli-Q grade) for five times.
- Transfer the surface-sterilized seeds on a sheet of moistened filter paper placed in a plastic Petri dish (Ø100 mm). For 50 seeds use 12-15 ml of sterilized water to moisten the filter paper.
- Place the seeds in a growth chamber set at 26 °C under 16 h light and 8 h dark condition for 1 week with a light intensity of approximately 701 mol/m2/sec illuminated by the daylight-white fluorescence lamp (FL40SEX-N-FL, NEC, Japan).

Figure 1. Rice root exudate treatment. A. One-week-old rice roots excised by scalpel were immobilized into agar block. The agar block was cut into small blocks (~2 x ~4 cm), each one contained around 20-25 mg of excised rice root. It was placed on P. japonicum plants maintained in vertical position for 2 weeks. B. Enlarged picture of (A). C. P. japonicum root without haustorium induction treatment; D and E. Induced haustoria (pointed by white arrows) formed along P. japonicum roots; F. Root tissues were cleared with chloral hydrate solution and observed under a microscope. G and H. The haustorium formed in a transgenic P. japonicum roots harbouring the construct ProPjYUC3:3xVENUS-N7-Pro35S:RFP, the merged filter(G) and YFP filter (H) photographs were taken under confocal Leica TCS-SP5 II microscopy. For details, see the reference Ishida et al. (2016). The white bars represent 1 cm and black bars 2.5 mm. - P. japonicum seed germination
- Place the seeds in a 1.5 ml plastic tube (maximum of ~100 seeds per tube).
- Sterilize the surface of seeds by immersing in 10% (v/v) commercial hypochlorite solution (~0.6% sodium hypochlorite in final solution). Place the tube in a vortex shaker set at maximum speed for 1 min. Replace the solution with new bleach solution and keep in a vortex shaker for 9 min.
- From this step, the procedures should be done in a sterile laminar flow hood, wash the seeds with sterile-deionized water for five times.
- Immerse the seeds in sterile-deionized water. Keep them in darkness at 4 °C for overnight.
- Use the 1 ml tip to sow seed one by one on the top of a square plate dish containing GM media, around 12-15 seeds per plate. Seal the square plates with a surgical tape and cover it with aluminium foil. Keep the seeds in darkness for three more days to stimulate germination.
- Remove partially the aluminium foil, keeping the root parts covered.
- Place the square dish vertically in a growth chamber set at 25 °C under 16 h light and 8 h dark condition for 2 weeks.
Note: It is important to let the parasitic plant to grow vertically to avoid the root to penetrate the agar media. - Haustorium induced by rice root exudate
- Excise the roots from 1-week-old rice seedlings, using sterilized forceps and scalpel on sterilized plate dish (Ø9 cm).
- Measure the root fresh weight. For 180 mg of fresh weight root tissues, 20-25 excised roots are necessary.
- In a sterilized plate dish (Ø9 cm), 180 mg of fresh weight rice roots and 5 ml water from the plate where rice seeds were germinated were placed.
- Using sterilized scalpel, cut the roots into small pieces (around ~1-3 mm of length).
- Add 20 ml of 0.8% (w/v) agar, warmed in a water bath set at 60 °C.
- Mix well until the root pieces are evenly distributed across the agar.
- Let the agar to solidify for 30 min.
- Cut the agar block to approximately ~2 x ~4 cm and place them on the parasitic plant roots growing on GM media. The size of agar block should be enough to cover the parasitic roots. (Figures 1A and 1B)
Note: When using transgenic roots, they are transferred to a plate dish (Ø9 cm) filled with 30 ml of 0.8% (w/v) agar with antibiotic cefotaxime (300 µg/ml). The agar block containing chopped rice roots and exudates should be put on the transgenic roots. - The haustorium will be formed within 24 h (Figures 1D to 1F). Examples of transgenic roots forming haustoria are shown (Figures 1G to 1H).
- Remove the agar containing rice roots and count the haustorium number under a stereomicroscope.
- Haustorium induced by DMBQ
- Dilute the 10 mM DMBQ stock solution to 10 µM DMBQ working solution in sterile-deionized water.
- Drop 7 ml (per plate) of 10 µM DMBQ solution on the top of the roots of 1-week-old P. japonicum grown in vertical position in a square plate. Seal the plate with a surgical tape (or Parafilm).
- Cover the root part with aluminium foil.
- Place the plates horizontally in a growth chamber set at 26 °C under 16 h light and 8 h dark condition, keeping the root part covered with aluminium foil.
- Haustoria will be formed along the parasite roots within 24 h. Count haustorium number under the stereomicroscope (Figure 2).
Note: P. japonicum roots are very sensitive to injury. If tissue damage is caused during the procedure, the percentage of formed haustorium might be altered.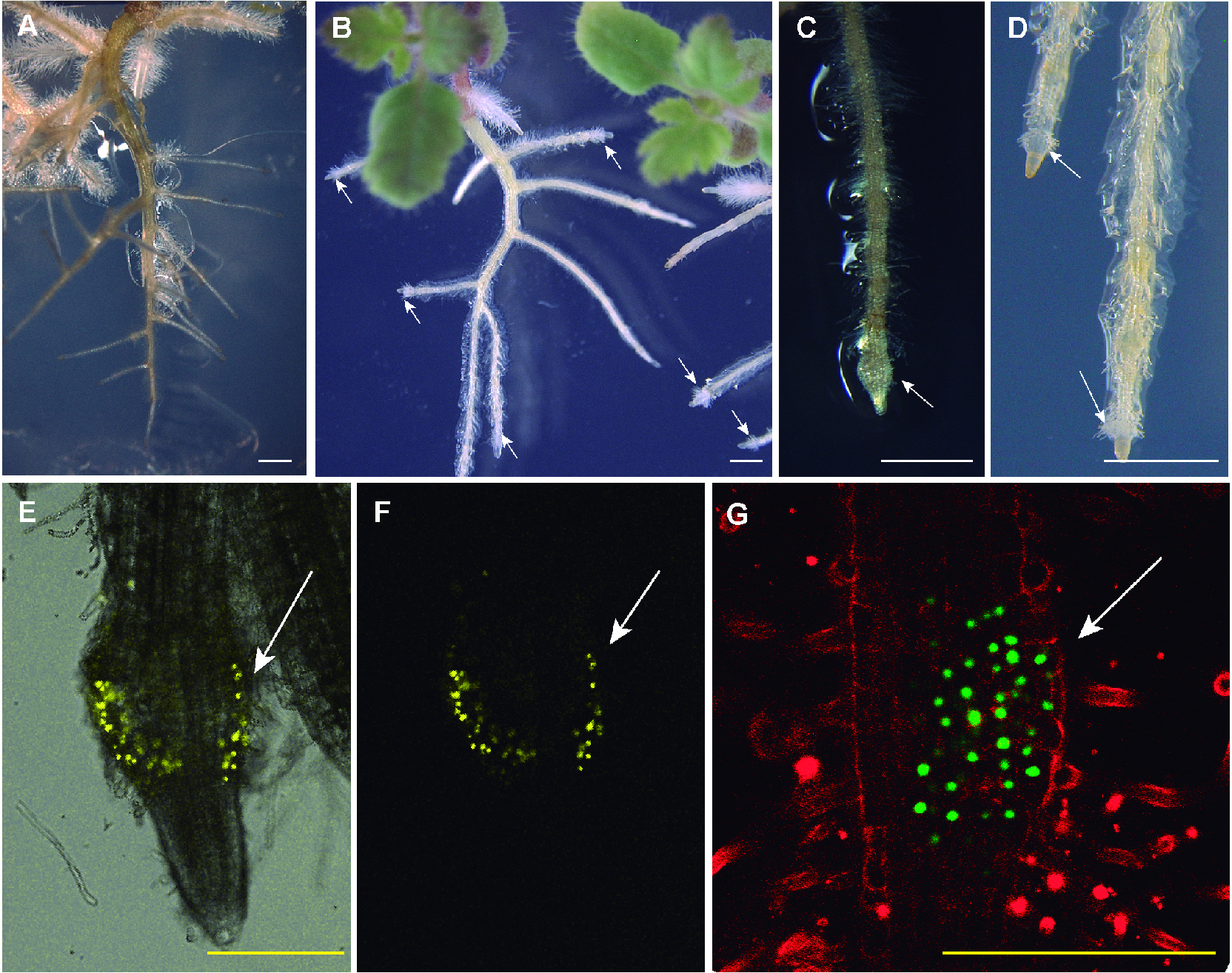
Figure 2. DMBQ-induced haustorium. A. Two-week-old P. japonicum growing in the absence of DMBQ; B. P. japonicum treated with 10 µM DMBQ; C and D. Enlarged pictures of (B). DMBQ-induced haustoria (pointed by white arrows) formed along P. japonicum roots. E to G. Transgenic P. japonicum roots photographs taken under confocal Leica TCS-SP5 II microscopy. A root transformed with the construct ProPjYUC3:3xVENUS-N7-Pro35S:RFP (Ishida et al., 2016) was observed under differential interference contrast filter and YFP filters. The merged picture (E) and the picture under YFP filter (F). Tissue carrying the construct CYCB1;2 pro::YFP was stained with 400 µg/ml propidium iodide to highlight root cell morphology. For details see the reference Ishida et al. (2011). Note that the haustoria developed in a rounded form with this method, which is distinct from the agar-based method described in Figure 1. The white bars represent 1 mm and the yellow bars 2.5 mm. - Clarification of root tissues with chloral hydrate*
- Immerse the root tissues in chloral hydrate solution overnight at room temperature.
- To examine the haustorium under microscope, place them on glass slide soaked in choral hydrate solution with the slide cover on top.
- Immerse the root tissues in chloral hydrate solution overnight at room temperature.
Data analysis
For accurate results we recommend performing at least three independent experiments, using 10 to 15 plants in each repetition. The plants with injured roots should be excluded from the analysis.
Observe the presence or absence of the haustorium in individual plant under a stereomicroscope and calculate the percentage of plants with the haustorium. Alternatively, count the number of formed haustoria per plant and analyse the average data for each repetition.
In the case of transformed transgenic roots, inspect the presence of haustorium under a stereomicroscope and determine the percentage of individual transgenic root with parasitic organ. Calculate the average haustoria per plant for each biological experiment.
Notes
The injured root compromised their ability to develop haustorium in P. japonicum. Thus, only healthy roots should be considered for data analysis.
Recipes
- GM media
1x Murashige and Skoog salts (Pre-mixed)
1% (w/v) sucrose
0.01% (w/v) Myo-inositol
0.06% (w/v) 2-(N-morpholino)ethanesulfonic acid (MES) monohydrate
0.8% (w/v) agar
Adjust pH to 5.7 with 1 N KOH
Sterilize it by autoclaving
For each square plate 100 ml of GM media was poured
The plates containing the media were stored at 4 °C for maximum 3-4 days - DMBQ stock solution (10 mM)
- Dissolve 16.8 mg of 2,6-dimethoxy-1,4-benzoquinone in 10 ml of dimethyl sulfoxide (DMSO)
- Aliquot the DMBQ solution in 1.5 ml of sterilized plastic tube
- Protect from light by covering with aluminium foil
- Store under -20 °C until the moment of use
- Chloral hydrate solution
8 g chloral hydrate
1 ml glycerol
2 ml deionized water
Store at room temperature until the moment of use
Acknowledgments
This protocol was adapted from our published work (Ishida et al., 2016) and from (Albrecht et al., 1999). This work was supported by MEXT KAKENHI grants (Nos. 24228008 and 15H05959 to K.S., Nos. 25114521, 25711019 and 904 25128716 to S.Y.) and the Ph.D. fellowship programs (MEXT to JKI).
References
- Albrecht, H., Yoder, J. I. and Phillips, D. A. (1999). Flavonoids promote haustoria formation in the root parasite triphysaria versicolor. Plant Physiol 119(2): 585-592.
- Chang, M. and Lynn, D. G. (1986). The haustorium and the chemistry of host recognition in parasitic angiosperms. J Chem Ecol 12(2): 561-579.
- Cui, S., Wakatake, T., Hashimoto, K., Saucet, S. B., Toyooka, K., Yoshida, S. and Shirasu, K. (2016). Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant Phtheirospermum japonicum. Plant Physiol 170(3): 1492-1503.
- Ishida, J. K., Wakatake, T., Yoshida, S., Takebayashi, Y., Kasahara, H., Wafula, E., dePamphilis, C. W., Namba, S. and Shirasu, K. (2016). Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell 28(8): 1795-1814.
- Ishida, J. K., Yoshida, S., Ito, M., Namba, S. and Shirasu, K. (2011). Agrobacterium rhizogenes-mediated transformation of the parasitic plant Phtheirospermum japonicum. PLoS One 6(10): e25802.
- Saucet, S. B. and Shirasu, K. (2016). Molecular parasitic plant-host interactions. PLoS Pathog 12(12): e1005978.
- Spallek, T., Mutuku, M. and Shirasu, K. (2013). The genus striga: a witch profile. Mol Plant Pathol 14(9): 861-869.
- Westwood, J. H., Yoder, J. I., Timko, M. P. and dePamphilis, C. W. (2010). The evolution of parasitism in plants. Trends Plant Sci 15(4): 227-235.
- Yoshida, S., Cui, S., Ichihashi, Y. and Shirasu, K. (2016). The haustorium, a specialized invasive organ in parasitic plants. Annu Rev Plant Biol 67: 643-667.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ishida, J. K., Yoshida, S. and Shirasu, K. (2017). Haustorium Induction Assay of the Parasitic Plant Phtheirospermum japonicum. Bio-protocol 7(9): e2260. DOI: 10.21769/BioProtoc.2260.
Category
Plant Science > Plant physiology > Biotic stress
Plant Science > Plant physiology > Phenotyping
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link