- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Growth Assay for the Stem Parasitic Plants of the Genus Cuscuta
Published: Vol 7, Iss 8, Apr 20, 2017 DOI: 10.21769/BioProtoc.2243 Views: 12329
Reviewed by: Andrea PuharSollapura J. VishwanathAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Efficient Inoculation Technique to Assess the Pathogenicity of Pantoea Species Associated to Bacterial Blight of Rice
Kossi Kini [...] Drissa Silue
Sep 5, 2020 5539 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2775 Views

Workflow for a Functional Assay of Candidate Effectors From Phytopathogens Using a TMV-GFP-based System
Peng Cao [...] Yuyan An
Apr 20, 2025 1724 Views
Abstract
Cuscuta spp. are widespread obligate holoparasitic plants with a broad host spectrum. Rootless Cuscuta penetrates host stems with so called haustoria to form a direct connection to the host vascular tissue (Dawson et al., 1994; Lanini and Kogan, 2005; Kaiser et al., 2015). This connection allows a steady uptake of water, assimilates and essential nutrients from the host plant and therefore enables Cuscuta growth and proliferation. To quantify the parasites’ ability to grow on potential host plants one can use the quantitative growth assay (Hegenauer et al., 2016) described herein, which exclusively utilizes fresh weight measurement as readout.
Keywords: Cuscuta reflexaBackground
In research fields of plant-pathogen resistance, either in basic research or in economic plant breeding, it is unavoidable to have an assay to quantify resistance against pathogen infection. To quantify the resistance/susceptibility of different plants against Cuscuta infections the simplest way is to measure the gain of biomass of Cuscuta growing on a plant of interest. This is a reliable method since Cuscuta is a holoparasite and its gain of biomass is completely depending on its ability to successfully infect another plant. Thus, unsuccessful infection of a plant leads to a decrease in biomass and subsequently the death of the parasite Cuscuta.
Materials and Reagents
- Gloves and lab suit (Cuscuta sap causes stains on skin and clothes)
- Mature Cuscuta (e.g., Cuscuta reflexa; see Note 1 for cultivation)
- Putative host plants
Equipment
- Weighing machine/balance (mass range between 0.01-100 g)
- Wooden planting rods (bamboo; diameter appropriate to the particular host plants stem diameter), available in gardening shops
- Scissor to cut Cuscuta shoots
Procedure
The whole experiment is performed under optimal conditions for the host plant. The optimal host plant conditions are provided by the seed supplier and can’t be generalized.
- Cut Cuscuta shoots (length ~15 cm) including the shoot tip from a mature plant and wind it around upright wooden sticks; be aware of winding direction (counter clockwise from bottom to top; Figure 1A) and integrity of the tip (see also Note 2). Keep the stick with the Cuscuta shoot in a vertical position for one day (e.g., by sticking it into Styrofoam, soil or sand). The Cuscuta shoot should hold by itself on the planting rod without contact to anything else (Figure 1A).
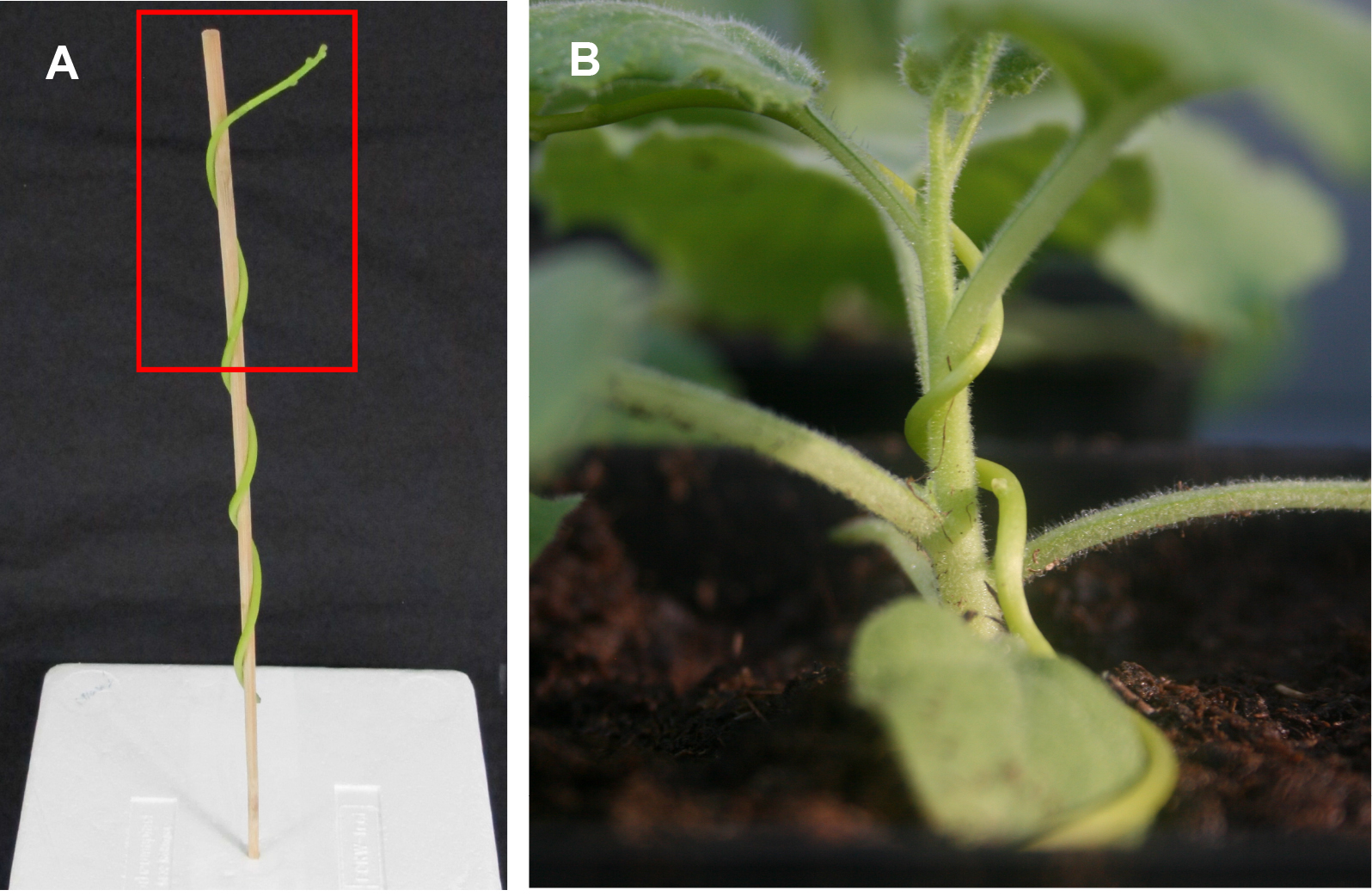
Figure 1. Starting the Cuscuta infection. A. Cuscuta reflexa shoot enwinding a wooden stick. Winding direction is counter clockwise from bottom to top. Red frame indicates the area where haustoria will most likely form. B. Cuscuta reflexa shoot wound around N. benthamiana plant after preconditioning around the wooden stick (A). - After one day (at the beginning of prehaustoria formation), when the shoot has been carefully uncoiled from the stick, weigh the shoots and transfer them to their host plants considering the winding direction (see also Note 3). The haustoria will form close to the shoot tip (Figure 1, red box) so make sure to wind this part around the host plant stems thoroughly. Remaining of the Cuscuta shoot will provide a source of nutrients and water until the haustoria connect with the host’s vascular system. Therefore, an equal length (e.g., 15 ± 1 cm) as well as an approximately same weight (e.g., 0.5-0.7 g) of the parasite shoots is relevant for reproducible results.
- After transfer (Figure 1B, see also Note 4), let the Cuscuta spp. shoots grow for the same time period (14-21 days). After that time of growth, remove individual shoots from the host plant and measure the fresh weight immediately for each individual.
Data analysis
Cuscuta’s ability and speed to accumulate biomass depends on the number of haustoria to acquire nutrients, and thus, on the time frame a single shoot needs to establish a successful haustorial connection to the host’s vascular tissue. For this reason, the variance of the Cuscuta growth can be occasionally high. A big number (> 10) of replicates and multiple repeats are recommended. The outcome of this experiment is the gain of biomass (in g) for every single Cuscuta shoot for the distinct timeframe. So resistance against Cuscuta (or susceptibility for the infection) can be quantified and compared by its ability to gain biomass on two distinct sets of host plants. A correction factor is not necessary if there is no great variance in the initial weight, never the less if this is the case it could be helpful to show the result as mass change (ΔFw = final shoot weight - initial shoot weight) during the time frame. This depends on the particular experiments experimenters will perform to answer their research questions.
The results can be presented as the mean of the Cuscuta shoot biomass 21 dpi (days post infection) of n replicates with standard deviation comparing two types of hostplants.
For the reduction of outlier effects, ranked data analysis and nonparametric tests like Mann-Whitney U-test can be used.
See also Note 5.
Notes
- Cultivation of Cuscuta: Cuscuta can be cultivated on many plants. In our Lab we use Coleus (Solenostemon scutellarioides) because it’s a robust plant and its red color gives a good contrast to spot Cuscuta shoots. You can easily cultivate Cuscuta when you place 6 to 10 host plants in close proximity so that Cuscuta can overgrow all of them. It’s important to concern that after Cuscuta overgrows the host plant it cannot be potted anymore, so make sure to provide enough nutrients for the host plant. Cuscuta should be cultivated under long day conditions with 17 h daylight. Cuscuta seeds and instructions how to start the culture can be requested in University botanical gardens or other research groups.
- The pre winding of Cuscuta around a wooden planting rod facilitates the later infection of the host plant due to an efficient prehaustoria formation, a clearly visible swelling of the shoot at the shoot-stick contact sites.
- It is important not to harm the Cuscuta shoot during the initial weight measurement and transfer to the host plant.
- In the first days of infection the Cuscuta shoot may search in the surrounding area of the host plant for other hosts despite infecting the plant it is sitting on. Therefore, the experimenter has to softly redirect the shoot back to its host.
- Blinded set up of the experiment: Comparison of e.g., N. benthamiana wild type plants with resistant transgenic CuRe1-expressing plants was performed as blind experiment in Hegenauer et al. (2016). One person randomly assigned numbers to each tested plant and a second person performed the infection with Cuscuta reflexa shoots.
- A successful infection of Coleus by Cuscuta reflexa is shown in Figure 2. At the side of haustorial infection Cuscuta is thickened (red frame). After successful infection Cuscuta starts growing and branching (blue arrow).

Figure 2. Coleus successfully infected by Cuscuta reflexa. Red frame: sides of haustorial development. Blue arrow: branching of Cuscuta.
Acknowledgments
The work was funded by DFG-grant (AL1426/1-2).
References
- Dawson, J. H., Musselman, L. J., Wolswinkel, J. P. and Dörr, I. (1994). Biology and control of Cuscuta. Weed Sci 6: 265-317.
- Hegenauer, V., Furst, U., Kaiser, B., Smoker, M., Zipfel, C., Felix, G., Stahl, M. and Albert, M. (2016). Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 353(6298): 478-481.
- Kaiser, B., Vogg, G., Furst, U. B. and Albert, M. (2015). Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front Plant Sci 6: 45.
- Lanini, W. T. and Kogan, M. (2005). Biology and management of Cuscuta in crops. Cienc Investig Agrar 32: 165-179.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hegenauer, V., Welz, M., Körner, M. and Albert, M. (2017). Growth Assay for the Stem Parasitic Plants of the Genus Cuscuta. Bio-protocol 7(8): e2243. DOI: 10.21769/BioProtoc.2243.
Category
Plant Science > Plant immunity > Disease symptom
Plant Science > Plant immunity > Disease bioassay
Cell Biology > Tissue analysis > Macroscopic observation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










