- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Melanoma Stem Cell Sphere Formation Assay
Published: Vol 7, Iss 8, Apr 20, 2017 DOI: 10.21769/BioProtoc.2233 Views: 15104
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Investigating Neural Stem Cell and Glioma Stem Cell Self-renewal Potential Using Extreme Limiting Dilution Analysis (ELDA)
Hong PT Nguyen [...] Theo Mantamadiotis
Sep 5, 2018 11571 Views

Thrombopoietin-independent Megakaryocyte Differentiation of Hematopoietic Progenitor Cells from Patients with Myeloproliferative Neoplasms
Chloe A. L. Thompson-Peach [...] Daniel Thomas
Jan 20, 2023 2364 Views

Isolation and Characterization of Cervical Cancer-Associated Mesenchymal Stem Cells From Primary Tumors Using Explant Culture
Surbhi Singla [...] Shalmoli Bhattacharyya
Jun 20, 2025 2775 Views
Abstract
Self-renewal is the ability of cells to replicate themselves at every cell cycle. Throughout self-renewal in normal tissue homeostasis, stem cell number is maintained constant throughout life. Cancer stem cells (CSCs) share this ability with normal tissue stem cells and the sphere formation assay (SFA) is the gold standard assay to assess stem cells (or cancer stem cells) self-renewal potential in vitro. When single cells are plated at low density in stem cell culture medium, only the cells endowed with self-renewal are able to grow in tridimensional clusters usually named spheres. In the recent years, SFA has also been used also to test the effect of several drugs, chemical and natural compounds or microenviromental components on stem cells self-renewal capacity. Here we will illustrate a detailed protocol to assess self-renewal of human melanoma stem cells, growing as melanospheres.
Keywords: MelanomaBackground
Cancer stem cells (CSCs) were first found in acute myeloma leukemia (Lapidot et al., 1994) and then were identified in many solid tumors including melanoma. CSCs are defined as cells strongly endowed with self-renewal and tumor initiating capacity, being able to regenerate the whole tumor heterogeneity in vivo. CSCs can be isolated from the tumor mass with different approaches based on phenotypic characteristic or biological properties, then their properties have to be tested in vitro (self-renewal) and in vivo (tumorigenic potential). Melanoma CSCs were isolated using a combination of cell surface markers, (Fang et al., 2005; Monzani et al., 2007; Schatton et al., 2008; Boiko et al., 2010; Boonyaratanakornkit et al., 2010) or through culture in specific stem cell media (Perego et al., 2010; Santini et al., 2012). To validate melanoma CSC self-renewal, and to study the effect of tumor microenvironmental factors on it (Tuccitto et al., 2016), we used the sphere formation assay (SFA) in vitro. Melanoma CSCs are plated at low density in stem cell culture medium and they grow in anchorage-independent, three-dimensional spherical structures, called melanospheres (tumorspheres, in general). Spheres forming efficiency is directly proportional to the number of melanoma CSCs present in the culture (one CSC corresponds to one melanosphere), thus giving a direct quantification of CSC amount in culture. This relatively simple method is useful to study the ability of any exogenous factors (growth factors, cytokines and chemokines, drugs) in perturbing CSC self-renewal (Tsuyada et al., 2012; Tuccitto et al., 2016). Here we provide detailed information about the SFA protocol we optimized in our laboratory for melanoma SFA.
Materials and Reagents
- Cell culture flask, area 150 cm2 (Corning, catalog number: 430823 )
- 15 ml Falcon tube (Greiner Bio One International, catalog number: 188261 )
- Micropipette P200 tips (Corning, catalog number: 4823 )
- 24-wells plates flat bottom (Corning, Costar®, catalog number: 3527 )
- 70 μm cell strainer (Corning, Falcon®, catalog number: 352350 )
- 15 ml polystyrene serological pipets (Corning, Falcon®, catalog number: 357551 )
- Melanospheres are obtained as described by Perego et al., starting from cell suspension obtained from melanoma surgical specimens or from previously established melanoma cell lines after culturing in stem cell medium (SCM) (Perego et al., 2010)
- RPMI
- 10% FBS
- Trypan blue solution, 0.4% (Sigma-Aldrich, catalog number: T8154 )
- SCM (see Recipes)
- DMEM:F-12, 1:1 mixture’ (Lonza, catalog number: BE12-719F )
- Epidermal growth factor (EGF) (PeproTech, catalog number: AF-100-15 )
- Basic fibroblast growth factor (bFGF) (PeproTech, catalog number: 100-18B )
- D-glucose (Sigma-Aldrich, catalog number: G7021 )
- Insulin (Sigma-Aldrich, catalog number: I6634 )
- Putrescine dihydrochloride (Sigma-Aldrich, catalog number: P5780 )
- Sodium selenite (Sigma-Aldrich, catalog number: S9133 )
- Progesterone (Sigma-Aldrich, catalog number: P6149 )
- Transferrin (Sigma-Aldrich, catalog number: T8158 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S8761 )
- 1x phosphate buffered saline (PBS) (Lonza, catalog number: 17-516 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A1933 )
- HEPES buffer (Lonza, catalog number: BE17-737 )
- L-glutamine (Lonza, catalog number: BE17-605E )
- Penicillin-streptomycin (Lonza, catalog number: 17-602E )
Equipment
- Automatic pipettor (PBI, catalog number: 857075 )
- Tissue culture incubator with CO2 input (Thermo Fisher Scientific, Thermo ScientificTM, model: FormaTM Series II 3110 )
- Centrifuge (Eppendorf, catalog number: 5810 )
- Hemocytometer (Marienfeld-Superior, Bürker, catalog number: 0640211 )
- Optical microscope (Carl Zeiss, model: Axiovert 25 )
Procedure
Notes:
- Work in tissue culture hood with sterile tools and equipment.
- This procedure was optimized for melanoma cells. The general principle could be applied to cancer stem cells for other tissues, with optimization.
- Prepare stem cell medium (SCM) and store it at 4 °C in the dark. SCM should be used within 3-4 weeks from preparation. It is also possible to prepare a concentrated solution (10x) with all the supplements (DMEM:F-12, 1:1 mixture supplemented with components listed above in the Materials and Reagents section from 6b to 6o). The concentrated medium can be stored at 20 °C up to 3 months. Growth factors (b-FGF and EGF) have to be added fresh directly to the medium.
Note: If you are planning to set up a sphere formation assay (SFA) with cells not cultured as melanospheres (adherent melanoma cells are grown in RPMI + 10% FBS, fresh melanoma tissue suspension is obtained from the processing of melanoma post-surgery specimens), start from step 10 of this procedure. In case of cells cultured with serum, be sure all the serum is removed (wash your cells extensively) before plating in SCM. - Collect melanospheres from the culture flask in a 15 ml Falcon tube.
- Centrifuge for 10 min at 30 x g at room temperature (RT).
- Discard the supernatant.
- Dissociate the pellet by pipetting up and down using a micropipette P200 tips for about 80-100 times in 100-200 μl of SCM.
Note: To dissociate the pellet, place the tip at the bottom of the tubes and pipette up and down fast enough to suspend the pellet and the cells without making bubble (too gentle pipetting will result in not complete sphere dissociation). In our hands, mechanical dissociation was the best method in terms of cell viability and results reproducibility to obtain single cell suspension. - Suspend the dissociated melanospheres in 2 ml SCM.
- Centrifuge for 10 min at 150 x g at room temperature (RT) and discard the supernatant.
- Dissociate the pellet by pipetting up and down using a micropipette P200 tips for about 80-100 times.
- Suspend the dissociated melanospheres in SCM and count.
Note: Make sure to obtain a single cell suspension. If clusters are still present, repeat steps 6-9. Eventually the cell suspension could be further filtered through a 70 μm cell strainer. - Take the required number of live cells (determined after counting with a hemocytometer in presence of trypan blue solution 0.4% to exclude dead cells) and adjust to a final concentration of 100 cells/100 µl in SCM.
- Pipette 400 µl/well of SCM in each 24-well plate for a total of 6 wells for each cell line or treatment.
Note: In our hands, SFA was largely used to test cytokines and chemokines effect on melanoma stem cells self-renewal (Tuccitto et al., 2016). We think that melanoma SFA could be optimized also to test the effects of drugs (targeting stem cells markers or active signaling pathways) or growth factors on melanoma stem cell self-renewal and or viability. - Seed 100 µl of the cells in SCM (100 cells/well) for each well, prepare at least 4-6 replicates for each condition. The seeded cells should appear as singlets (see Figure 1A).
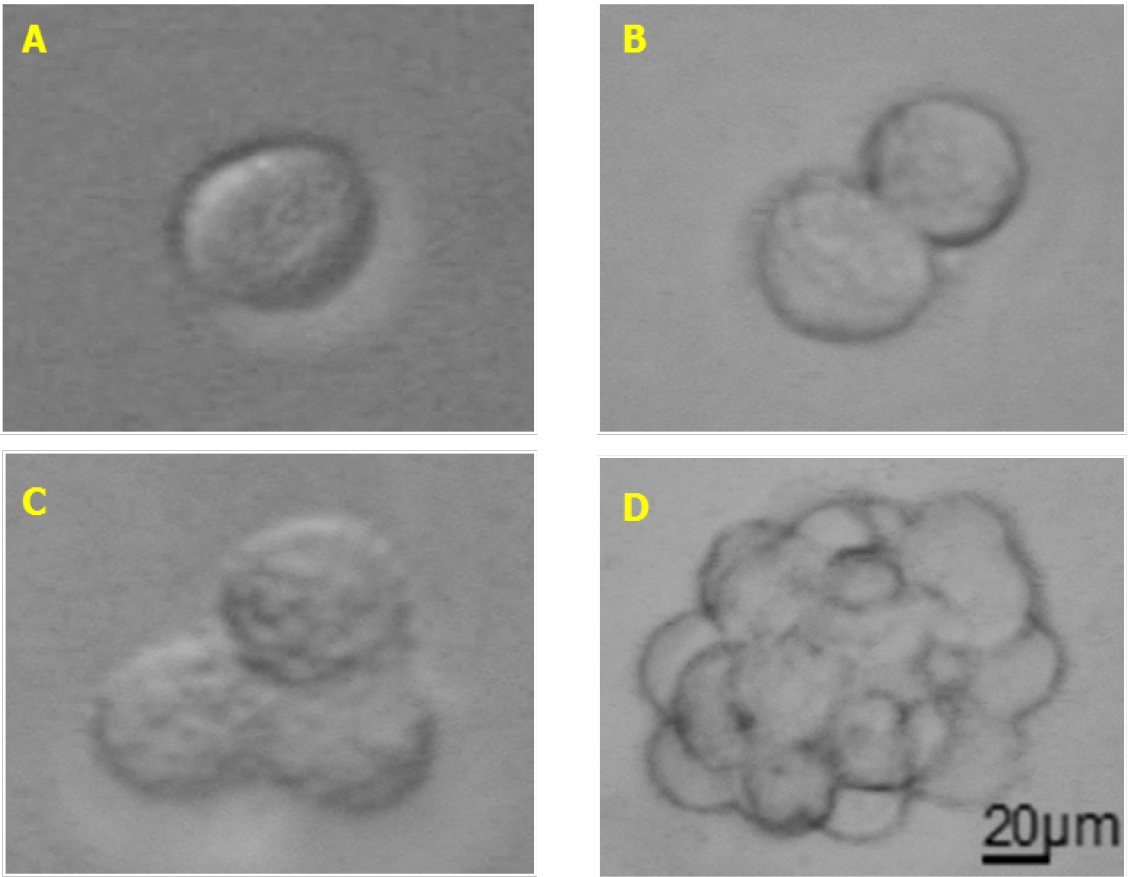
Figure 1. Melanoma sphere formation. A. Single cell melanospheres plated in SCM; B. Doublets appearing when cells start dividing; C. Triplets formed during cell proliferation; D. Fully formed melanospheres. Total magnification 200x (A-D), scale bar = 20 µm. - Place the plate in an incubator set at 37 °C with 5% CO2.
- 24-72 h after seeding, the cells doublets and triplets will appear in wells and could be counted under an optical microscope (see Figures 1B and 1C).
Note: If the media turns yellow, add fresh media to the well without dissociating or touching the ongoing forming spheres. - Melanospheres will be completely formed in each well 5-8 days after seeding and can be counted again under an optical microscope with 10x objective (total magnification 100x) analyzing the entire well. If spheres are bigger enough to be clearly distinguished from monolayer aggregates and single cells, also lower magnification should be used (20-50x total magnification). For better results, count melanospheres when they are clearly visible and do not let them over-proliferate. If this happen, melanospheres could attach to the plate and reliable counting will be difficult.
Note: When melanospheres are formed (see Figure 1D) it is difficult to distinguish the exact number of cells due to their compact 3D structure. Occasionally, some monolayer aggregates of about 10-30 cells could be observed, but those aggregates shouldn’t be accounted as melanospheres (Figure 2). Generally, spheres could be counted around day 5 up to day 10 from SFA seeding, optimal counting time should be determined for each cell line or sample and then maintained constant across different experiments. To simplify the count of the spheres it could be useful drawing some line with a marker to subdivide the well into quarters.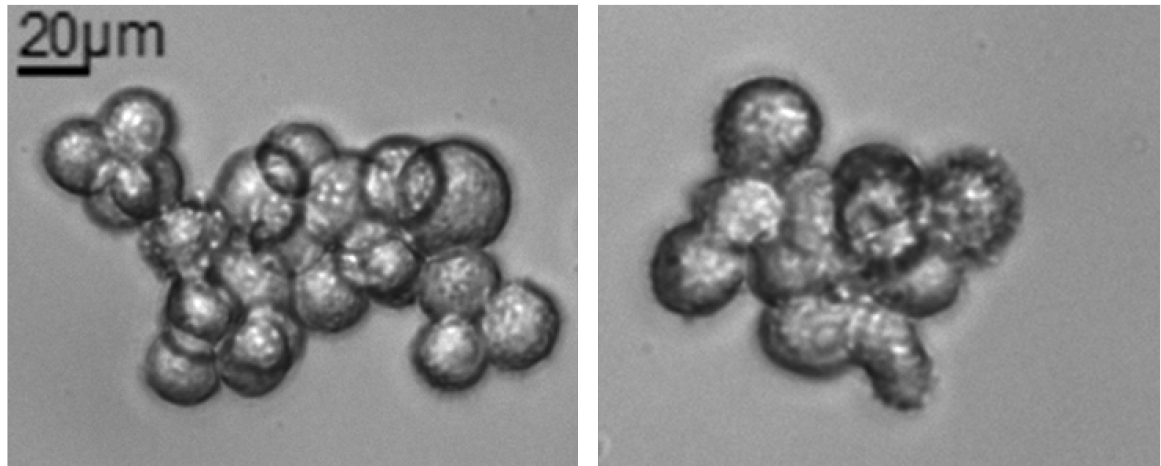
Figure 2. Non-melanosphere clusters. Example of non-spherical cluster that could be found in SFA. Those clusters could not be counted as spheres. Total magnification 200x, scale bar = 20 µm.
Data analysis
Sphere efficiency can be reported as percentage (%) of spheres formed dividing by the original number of cells seeded or number of spheres counted considering the total number of single cells seeded. We usually presented the results as mean ± SD (or SEM) of biological replicates. Each cell line was tested multiple times to be sure that the sphere forming efficiency was maintained after extensive cell culture.
Notes
- Self-renewal efficiency it is cell-type or cell-line dependent, depending on the melanospheres origin. In our hands, melanospheres showed between 30% and 15% of sphere forming efficiency (Perego et al., 2010; Tuccitto et al., 2016).
- Self-renewal efficiency is generally higher for cells maintained in culture as melanospheres than for cells previously cultured in regular melanoma culture medium with serum (adherent melanoma cultures). Melanospheres cultures are already enriched in CSC compared to adherent culture, so SFA from adherent cell lines would result in lower sphere forming efficiency, in general.
Recipes
- Stem cell medium (SCM) (500 ml)
DMEM:F-12 1:1 mixture
10 ng/ml EGF
5 ng/ml bFGF
600 µg/ml D-glucose
5 µg/ml insulin
1 µg/ml putrescine dihydrochloride
0.01 µg/ml sodium selenite
0.01 µg/ml progesterone
20 µg/ml transferrin
0.11 µg/ml NaHCO3
4 mg/ml BSA
119.2 µg/ml HEPES buffer
292 µg/ml L-glutamine
200 UI/ml penicillin-streptomycin
Notes: - 10x BSA solution in 1x PBS can be stored at -20 °C.
- If stock solution are made, make sure to respect the final volume of 500 ml, otherwise some components will be diluted and the media should not work.
Acknowledgments
This protocol was used in Perego et al., 2010 and Tuccitto et al., 2016. These works were supported by the Associazione Italiana Ricerca sul Cancro (CC IG-10615).
References
- Boiko, A. D., Razorenova, O. V., van de Rijn, M., Swetter, S. M., Johnson, D. L., Ly, D. P., Butler, P. D., Yang, G. P., Joshua, B., Kaplan, M. J., Longaker, M. T. and Weissman, I. L. (2010). Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466(7302): 133-137.
- Boonyaratanakornkit, J. B., Yue, L., Strachan, L. R., Scalapino, K. J., LeBoit, P. E., Lu, Y., Leong, S. P., Smith, J. E. and Ghadially, R. (2010). Selection of tumorigenic melanoma cells using ALDH. J Invest Dermatol 130(12): 2799-2808.
- Fang, D., Nguyen, T. K., Leishear, K., Finko, R., Kulp, A. N., Hotz, S., Van Belle, P. A., Xu, X., Elder, D. E. and Herlyn, M. (2005). A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65(20): 9328-9337.
- Lapidot, T., Sirard, C., Vormoor, J., Murdoch, B., Hoang, T., Caceres-Cortes, J., Minden, M., Paterson, B., Caligiuri, M. A. and Dick, J. E. (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464): 645-648.
- Monzani, E., Facchetti, F., Galmozzi, E., Corsini, E., Benetti, A., Cavazzin, C., Gritti, A., Piccinini, A., Porro, D., Santinami, M., Invernici, G., Parati, E., Alessandri, G. and La Porta, C. A. (2007). Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer 43(5): 935-946.
- Perego, M., Tortoreto, M., Tragni, G., Mariani, L., Deho, P., Carbone, A., Santinami, M., Patuzzo, R., Mina, P. D., Villa, A., Pratesi, G., Cossa, G., Perego, P., Daidone, M. G., Alison, M. R., Parmiani, G., Rivoltini, L. and Castelli, C. (2010). Heterogeneous phenotype of human melanoma cells with in vitro and in vivo features of tumor-initiating cells. J Invest Dermatol 130(7): 1877-1886.
- Santini, R., Vinci, M. C., Pandolfi, S., Penachioni, J. Y., Montagnani, V., Olivito, B., Gattai, R., Pimpinelli, N., Gerlini, G., Borgognoni, L. and Stecca, B. (2012). Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells 30(9): 1808-1818.
- Schatton, T., Murphy, G. F., Frank, N. Y., Yamaura, K., Waaga-Gasser, A. M., Gasser, M., Zhan, Q., Jordan, S., Duncan, L. M., Weishaupt, C., Fuhlbrigge, R. C., Kupper, T. S., Sayegh, M. H. and Frank, M. H. (2008). Identification of cells initiating human melanomas. Nature 451(7176): 345-349.
- Tsuyada, A., Chow, A., Wu, J., Somlo, G., Chu, P., Loera, S., Luu, T., Li, A. X., Wu, X., Ye, W., Chen, S., Zhou, W., Yu, Y., Wang, Y. Z., Ren, X., Li, H., Scherle, P., Kuroki, Y. and Wang, S. E. (2012). CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res 72(11): 2768-2779.
- Tuccitto, A., Tazzari, M., Beretta, V., Rini, F., Miranda, C., Greco, A., Santinami, M., Patuzzo, R., Vergani, B., Villa, A., Manenti, G., Cleris, L., Giardiello, D., Alison, M., Rivoltini, L., Castelli, C. and Perego, M. (2016). Immunomodulatory factors control the fate of melanoma tumor initiating cells. Stem Cells 34(10): 2449-2460.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tuccitto, A., Beretta, V., Rini, F., Castelli, C. and Perego, M. (2017). Melanoma Stem Cell Sphere Formation Assay. Bio-protocol 7(8): e2233. DOI: 10.21769/BioProtoc.2233.
Category
Cancer Biology > Cancer stem cell > Cell biology assays > Self-renewal
Stem Cell > Adult stem cell > Cancer stem cell
Cell Biology > Cell-based analysis > Non-adherent culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link