- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
The Object Context-place-location Paradigm for Testing Spatial Memory in Mice
Published: Vol 7, Iss 8, Apr 20, 2017 DOI: 10.21769/BioProtoc.2231 Views: 12814
Reviewed by: Xi FengEdgar Soria-GomezSoyun Kim

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2607 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1645 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2585 Views
Abstract
This protocol was originally designed to examine long-term spatial memory in PKMζ knockout (i.e., PKMζ-null) mice (Tsokas et al., 2016). Our main goal was to test whether the ability of these animals to maintain previously acquired spatial information was sensitive to the type and complexity of the spatial information that needs to be remembered. Accordingly, we modified and combined into a single protocol, three novelty-preference tests, specifically the object-in-context, object-in-place and object-in-location tests, adapted from previous studies in rodents (Mumby et al., 2002; Langston and Wood, 2010; Barker and Warburton, 2011). During the training (learning) phase of the procedure, mice are repeatedly exposed to three different environments in which they learn the spatial arrangement of an environment-specific set of non-identical objects. After this learning phase is completed, each mouse receives three different memory tests configured as environment mismatches, in which the previously learned objects-in-space configurations have been modified from the original training situation. The mismatch tests differ in their cognitive demands due to the type of spatial association that is manipulated, specifically evaluating memory for object-context and object-place associations. During each memory test, the time differential spent exploring the novel (misplaced) and familiar objects is computed as an index of novelty discrimination. This index is the behavioral measure of memory recall of the previously acquired spatial associations.
Keywords: SpaceBackground
The behavioral neuroscientist’s toolkit of laboratory rodent behaviors has needed to expand to both propel and keep up with the progress of the program to identify the molecular mechanisms of memory. The traditional approach has been to use reinforced behaviors to control learning and formation of memory and limit behavior to readily quantified endpoint measures. However, to meet the growing sophistication of the learning and memory field and to test the generality of the postulated mechanisms, the toolkit has needed to expand to multiple estimates of memory. It has been especially important to use memory assays that minimize the need for behavioral shaping (a form of learning itself) and the manipulation of other motivational factors, many of which potentially elicit stress and other potential confounds of the molecular basis of memory (Lesburguères et al., 2016).
Unlike behavioral paradigms that rely on conditioned responses, novelty-preference tasks exploit the rodent’s spontaneous exploratory behavior and its innate tendency to investigate instances of change (novelty) in a familiar environment (Steckler et al., 1998a). The basic reasoning is that memory for the familiar condition is estimated by the extent to which behavior differs in response to novelty. Accordingly, the fundamental principle of a novelty-preference memory paradigm is to experimentally create a ‘non-matching’ condition between the learning (or encoding) phase and the memory test, such that the animal will express its memory of the original learning experience by preferentially exploring the novel stimuli over the familiar ones.
Novelty-preference tasks are easy-to-use and offer a great versatility for investigating cognitive functions such as spatial memory in rodents (Kinnavane et al., 2015). The following protocol is inspired by previous studies that have used different spatial versions of the novelty-preference paradigm, specifically the object-in-place, object-in-context and object-location task variants (Mumby et al., 2002; Langston and Wood, 2010; Barker and Warburton, 2011). Typically, these task protocols consist of a short learning phase followed by a memory retention test, during which the original spatial configuration of the environment is modified in a specific way. In prior studies however, the different task variants have been presented separately, requiring independent groups of animals to be tested. In addition, the tests are often at short delays (minutes to hours) rather than days, as required to specifically evaluate mechanisms of long-term memory persistence. In the present experimental design each memory retention test is performed to evaluate memory lasting at least 24 h in the same animal and corresponds to a specific spatial manipulation of the original learning experience. Additionally, by varying the number of objects that are presented during the learning phase (i.e., 4 objects versus 2 objects), the following protocol allows direct manipulation of the amount of information to be remembered, not only the type of information.
Here we present the behavioral protocol that was recently used to assess the molecular mechanisms of 24-h long-term memory persistence using wild-type and PKMζ knockout mice (Tsokas et al., 2016). The protocol was sensitive enough to reveal that the molecular mechanisms that are crucial for object-in-place associations amongst four objects require the persistent kinase PKMζ, whereas non-PKMζ dependent mechanisms are sufficient for the maintenance of object-in-context associations and object-location associations involving two objects. A similar dissociation between the neural mechanisms that support object-in-place and object-in-context associations is also observed at the level of the dorsal hippocampus. In particular, permanent or temporary lesion of dorsal hippocampus is sufficient to impair acquisition of object-in-place associations but not object-in-context associations (Langston and Wood, 2010; reviewed by Langston et al., 2010), with a potentially greater role of the dentate gyrus-CA3 subcircuit (Lee et al., 2005). Object-in-context associations seem to critically depend on postrhinal cortex function (Norman and Eacott, 2005) whereas object-location associations tend to be sensitive to hippocampus manipulations (Hardt et al., 2010; Barker and Warburton, 2011). Although these dissociations suggest sharp dependencies of the cognitive function each test evaluates on distinct brain regions, the boundaries of these structure-function associations may not be definitive, because the consequences of the lesions depend on task parameters, including whether contextual cues are local or distal and whether the role of a structure is being evaluated by tests of memory acquisition and consolidation or tests of memory retention (reviewed by Langston et al., 2010). Indeed, recognition memory, a subset of which is assessed by the object context-place-location paradigm that we here describe, appears to be mediated by two extended networks of structures one including the hippocampus that is specialized for spatial recognition memory and the other including postrhinal cortex that is specialized for non-spatial/item recognition memory (reviewed by Steckler et al., 1998b).
Materials and Reagents
- 3 open boxes (31 x 31 x 19 cm, L x W x H). They are large, solid-bottom, polysulfone cages purchased from Thoren Caging Systems (Hazelton, PA)
- 3 different sets of laminate sheets displaying patterns of black shapes on white background (see Figure 1).
- 10 non-identical objects
Note: We used a combination of plastic toys (PetCo®, USA), flasks and jars that differ in shape and/or materials that are difficult to chew, such as strong natural rubber, Pyrex®, polypropylene, and aluminum. These are the objects that the mice will explore. Each object was unique but had approximately equal size, and they were tall enough to prevent the mice from climbing on the objects. The footprint dimension was approximately 6 cm and the height was 17 cm. The objects must be removable and should be easily washed (see Figure 1) - Wild-type male adult (4-month old) mice (C57BL6/J, THE JACKSON LABORATORY)
Notes: - In the original publication of this protocol, we have also used adult mutant male mice from the PKMζ knockout mouse line, (PKMζ-null, originally described in Lee et al., 2013) which were bred from breeders provided by Robert O. Messing (University of Texas, Austin, TX).
- Mice were housed individually, in an environment with controlled temperature (23 °C) and humidity, under a 12-12 h light-dark cycle with ad libitum access to food and water. All behavioral procedures were conducted during the 7 AM-7 PM light phase of the cycle. While it was not tested, training and testing with a reverse light cycle should have no impact on the behavioral outcomes, as long as the housing and training conditions are consistent. Mice were housed individually in dual cages (2 individual compartments per cage; 1 Wild Type and 1 Knockout per cage). The mutant animals were bred in our facility, and the genotype is known when the animals are weaned. Other mice in the study were housed individually because they were implanted with intracerebral injection cannulae or microelectrode arrays that can be damaged by another mouse. Consequently, we opted for individual housing to ensure similar housing conditions between all animals. Alternative housing, such as in pairs, might be preferable when practical.
- Water and 70% EtOH
Equipment
- Apparatus
The experiments are conducted in the 3 open plastic boxes, customized with different patterns in order to make 3 distinct contexts (A, B and C). For each context, 3 of the 4 inside walls are covered by laminate paper sheets displaying a specific pattern with a strong contrast so that the contexts can be easy to discriminate for the mice. We created walls with a repeating pattern of a black shape on white background (either stripes or dots, see Figure 1) for two of the contexts, and used an all white laminate for the third context. The fourth wall is always left transparent and in the south position, to provide an orientation cue in each context. Each box is placed at the center of the experimental room on an elevated support.
Figure 1. Example of the three different training contexts A, B and C with their specific set of objects. Upper panel: three of four walls are covered with a specific visual pattern. The fourth wall at the south is always left clear. Lower panel: the objects are non-identical and each context contains a unique set of objects. Note that the bright lighting as captured in these pictures is only for illustration, lighting should be dim, to be optimally comfortable for the mice and allow them to see and discriminate the objects. A 10-15 lux light intensity is recommended. - Objects
The objects (4 objects/context for A and B, 2 objects for context C) are fixed on the floor with removable adhesive putty such that their edges are 5 cm away from the walls. The precise position of each object is always the same (see Notes for additional comments). To ensure that the objects are repositioned in the same configuration after cleaning between trials, we recommend marking the position on the bottom of both the objects and the box. - Overhead camera (Firefly USB 2.0 camera, FLIR Integrated Imaging Solutions, catalog number: FMVU-03MTM-CS ), equipped with ½” 4-12 mm CCTV Lens (TAMRON, catalog number: 12VM412ASIR ) and software (Tracker, Bio-Signal Group, Acton, MA) for digital video tracking of the mouse’s position and recording the overall behavior on video
- Tracking system
We used a PC-controlled video tracking system (Tracker, Bio-Signal Group, Acton, MA) to accurately detect the position of the animal in each context during the behavioral sessions and to record its exploratory behavior for offline analysis. The position of the mouse was taken to be the centroid of its image in each 1/30 sec video frame.
Procedure
- One week prior to the behavioral experiments, each day, all animals are familiarized to the transportation procedure from the housing location to the experimental room. The animals are also handled in the experimental room by the experimenter to habituate them to the procedure.
- Each day, mice are placed in the experimental room 30 min before the beginning of the behavioral experiments for acclimation.
- The duration of the entire behavioral procedure is about 9 consecutive days. As depicted below in Figure 2, each mouse is trained and tested in the object-context-place tasks first, followed by the object-location task. Each task procedure consists of three phases: pre-training, training and retention test.
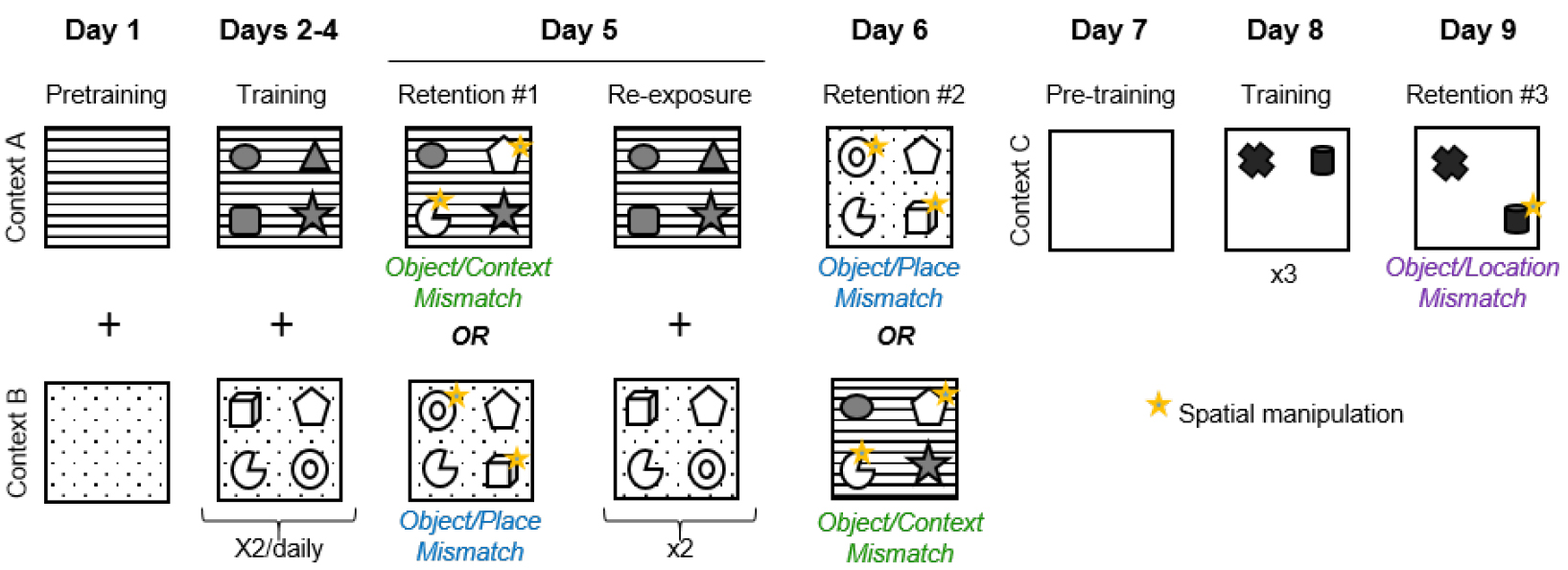
Figure 2. Experimental design of the object context-place-location paradigm - Day 1: Pretraining in contexts A and B
- Animals are habituated to contexts A and B. They explore each box for 10 min with no objects present. Each pretraining session is separated by a 1-h inter-trial interval. The pretraining session starts immediately after the animal has been placed, by hand, at the center of the box, its nose facing the south wall.
- Between each pretraining session, the boxes and objects are cleaned with water followed by 70% EtOH, which is allowed to dry. This is done to prevent build up of olfactory cues.
- At the end of the 10 min of exploration the animal is removed from the context and returned to its home cage.
- Animals are habituated to contexts A and B. They explore each box for 10 min with no objects present. Each pretraining session is separated by a 1-h inter-trial interval. The pretraining session starts immediately after the animal has been placed, by hand, at the center of the box, its nose facing the south wall.
- From Day 2 to Day 4: Training in contexts A and B
The mice are allowed to explore contexts A and B, each during two 5-min trials/day, separated by a 1-h inter-trial interval. These trials allow the mice to learn the spatial arrangement of the four objects that are associated with each context.
Note: The order of each context exploration is counterbalanced between training days and between animals within each experimental group (Table 1).
Table 1. Example of counterbalancing the order of context exploration (i.e., Contexts A vs. B) between training days and the order within each experimental group (i.e., Wild Type vs. PKMζ-null in the original study)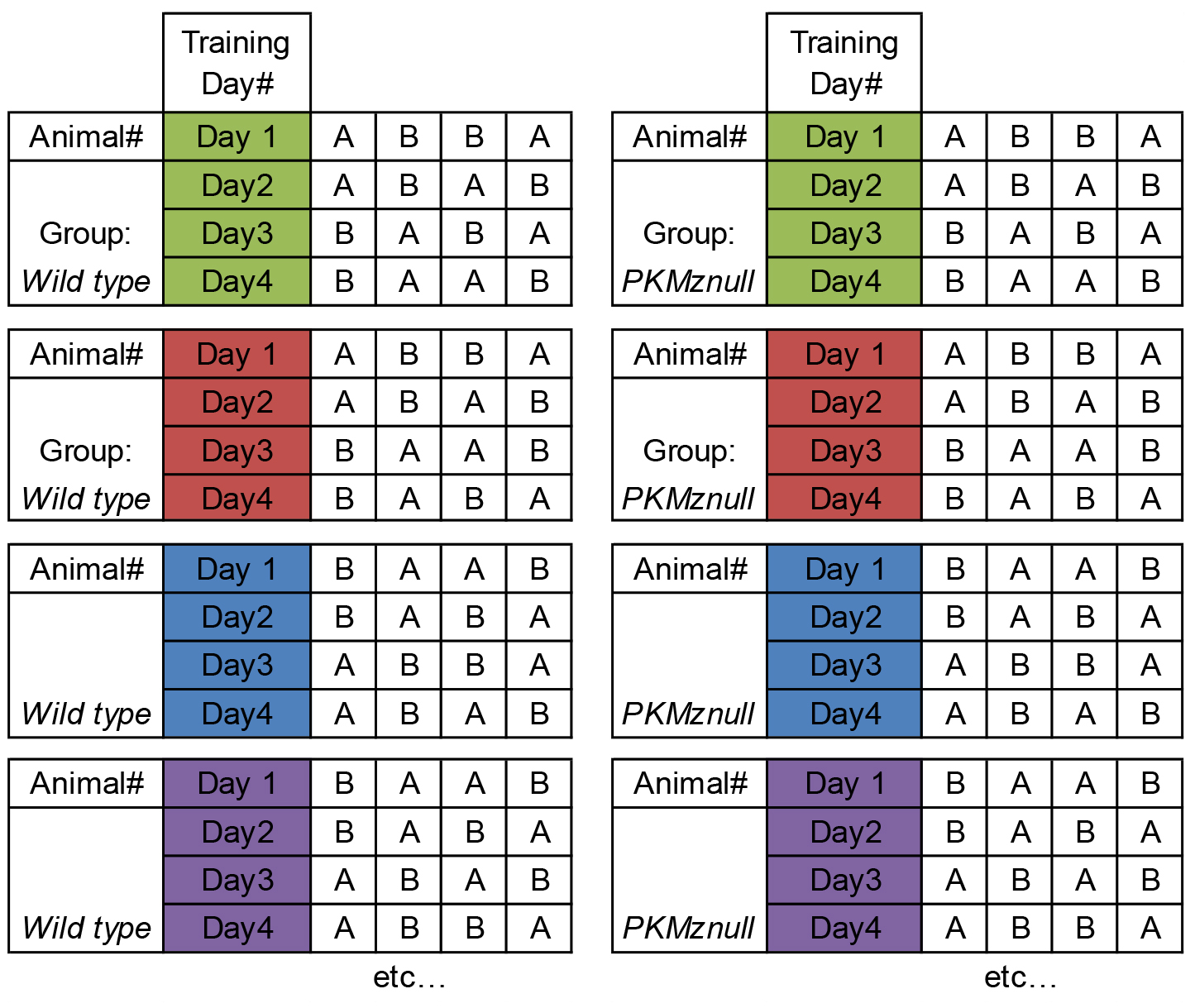
- Day 5: Retention test #1
Mice are given a first memory retention test that is either an object/context or an object/place mismatch test. In the object/context mismatch test, two of the four objects that had only been encountered in one context are placed in the second context, whereas in the object/place mismatch test two objects from one four-object configuration are place-permuted in the same context they had previously been encountered. The animal is allowed to explore for 3 min. The 3-min duration of the retention test session has been carefully validated. After 3 min the mice become familiar with the novel object configuration and so spatial novelty triggered by the object permutation is no longer detected by the animal. The mice will subsequently explore all the objects equally, whether or not there is a mismatch. - Day 5: Re-exposure
The same day, an hour after the memory retention test, the mice receive an additional training session (5-min exploration twice in each context A and B) in order to minimize the potential effects of learning the mismatch object-space associations that might have been induced by the retention test. - Day 6: Retention test #2
Mice are given a second 3-min memory retention test, either an object/context or an object/place mismatch test, whichever test was not administered on Day 5.
Note: The order of the retention tests on Days 5 and 6, the objects and the places that are permuted, are counterbalanced between animals within each experimental group (Figure 3).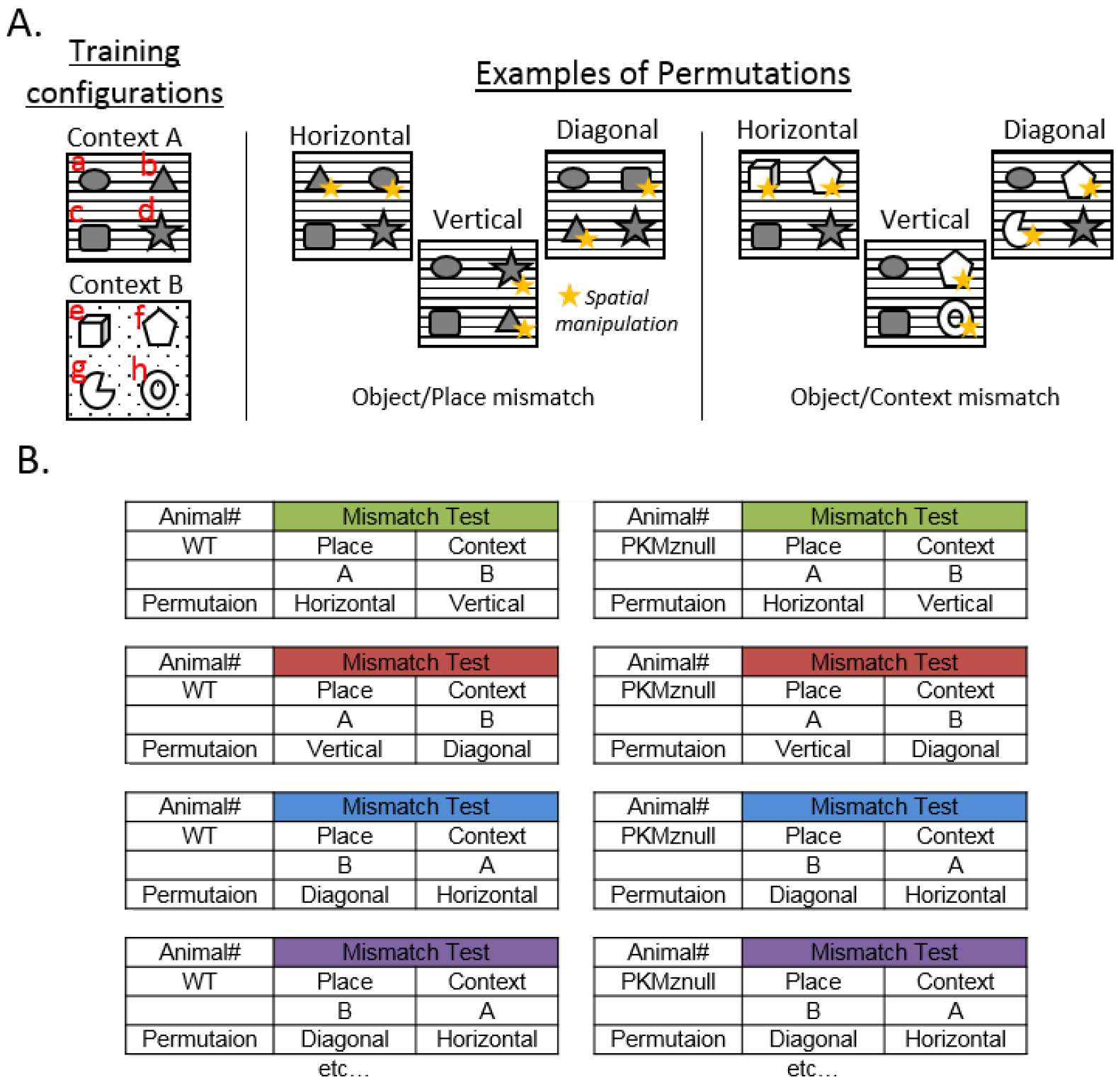
Figure 3. Counterbalancing Object and/or Place permutations and Retention Test order. A. Examples of spatial-object counterbalancing by making object pair permutations in the Object/Place and Object/Context mismatch tests. Because each subject can have an idiosyncratic preference or dislike for a particular object and/or context, it is important to counterbalance the object-context presentations amongst the subjects within each group during memory tests. There are a very large number of permutations possible. Consider randomizing the objects within a context by exchanging pairs of objects along the horizontal, vertical and two diagonals to generate six unique patterns of permutations and exchanging half of the objects from each context, which will generate 12 object-context arrangements. The six examples of objects permutations represented above from the initial training configuration (the training configuration is displayed as a-b-c-d) in Context A are b-a-c-d (horizontal), a-d-c-b (vertical), a-c-b-d (diagonal); they illustrate counterbalancing of the spatial manipulations for the Object/Place mismatch test. Configurations e-f-c-d (horizontal), a-f-c-h (vertical), a-f-g-d (diagonal) illustrate examples of counterbalancing spatial manipulations for the Object/Context mismatch test. B. Example of counterbalancing the order of the retention tests on Days 5 and 6 (i.e., Object/Place vs. Object/Context mismatch tests) and the type of object permutations between animals within each experimental group (e.g., Wild Type vs. PKMζ-null). - Day 7: Pretraining in context C
Mice are habituated to explore a new context, context C, for 10 min. - Day 8: Training in context C
Mice are allowed to explore context C for three sessions of five minutes separated by 1-h inter-trial interval, to learn the spatial configuration of two new objects in a novel environment. - Day 9: Retention test #3
Mice are given a 3-min retention trial consisting of an object/location mismatch test, in which the location of one object is changed.
Note: The relocated object and its relative relocation in the context are also counterbalanced between animals within each experimental group. - Behavioral measures of memory performance
Each video is analyzed offline to manually score the time the mouse is engaged in exploration of an object for each of the retention tests. Object exploration is defined as the nose of the animal being oriented toward the object at a distance of < 2 cm. Each video is therefore replayed in the Tracker software using a 2 cm wide annular mask around each object to define the object exploratory area. Within this area, animal’s activity such as sniffing or touching the object with paws is counted as object exploratory activity only if the animal’s nose is orientated toward the object (see Notes). Measuring object exploration is performed by an experimenter who is blind to the animal’s experimental group and whether the objects have been changed. Memory performance in the three different memory tests are quantified and analyzed using a discrimination index calculated as the absolute difference in time spent exploring the changed (i.e., incorrect, misplaced or relocated) objects and the unchanged objects divided by the total time spent exploring all the objects. As such, the index takes into account individual differences in the total amount of exploration. Good memory retention corresponds to a positive discrimination index, which reflects that the animal was spending more time exploring the incorrect (object/context mismatch), displaced (object/place mismatch) or relocated (object/location mismatch) objects than the objects that had remained unchanged (Figure 4).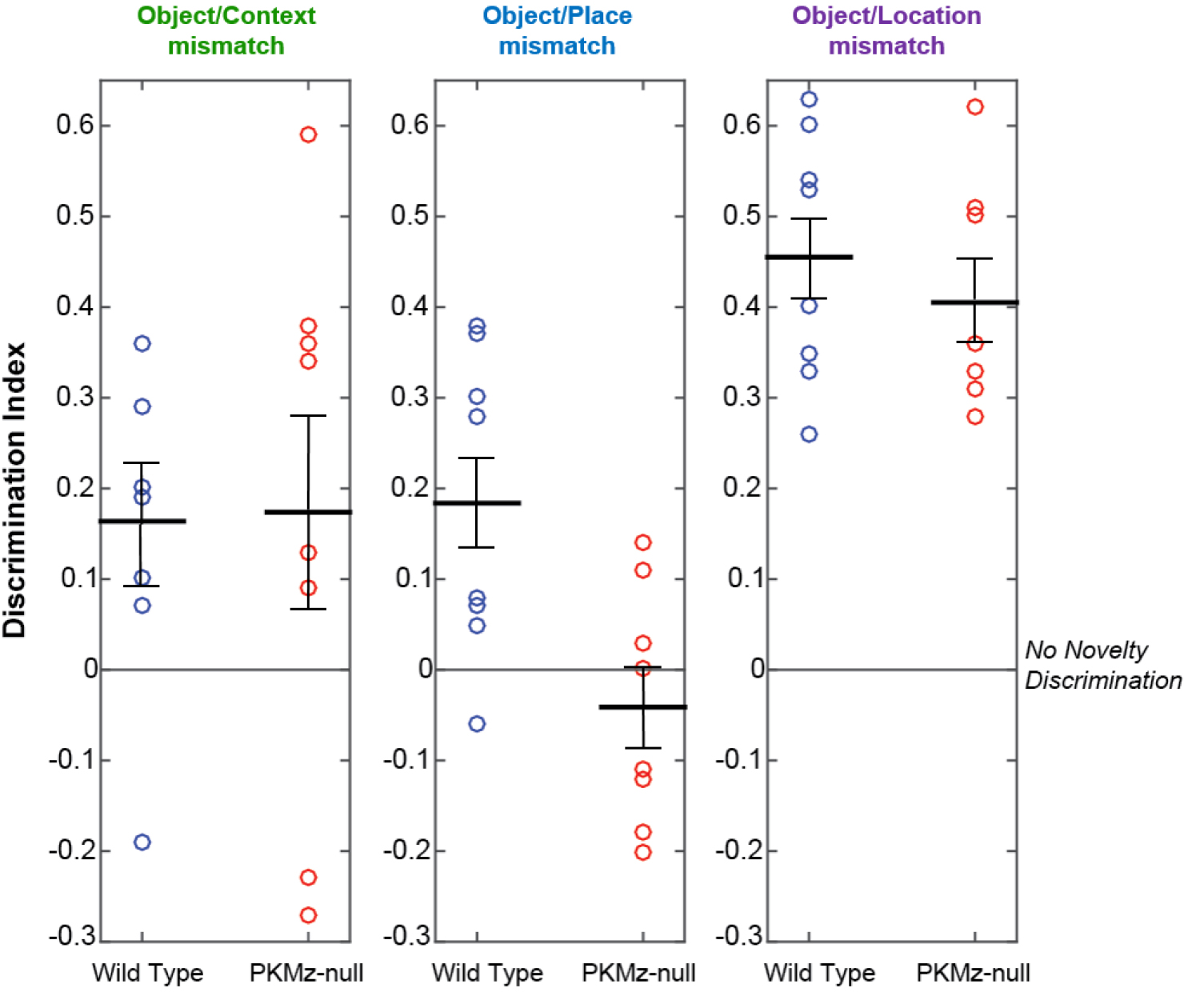
Figure 4. Representative data of behavioral performance. Data can be represented as separate dot plots for each test, depicting the distribution of individual memory performance within each group (e.g., Wild type vs. PKMζ-null). Black bars: Mean ± SEM. The graph is adapted from the data in Tsokas et al., 2016.
Data analysis
Memory performance is analyzed using a one-way ANOVA with repeated measures. The individual effects of the Independent Factor (Group) and the Within-Subjects Factor (Retention test), as well as the Interaction and post-hoc tests are considered significant at an alpha level of 0.05.
Notes
- To make each to-be-explored object unique and identifiable, we recommend using a variety of shapes and type of materials. The choice of the set of objects should be validated prior to the experiment by testing each configuration set with mice in a pilot study to avoid obviously biased preference to investigate one object over the others.
- Animals should not be able to climb the objects, because it would affect the measurement accuracy of the exploratory activity.
- The lighting of the objects should be homogenous to avoid creating shadows at the corners of the experimental box. Dark corners are typically preferred by mice and the object-biased presence of preferred places could therefore induce a place preference that could interfere with the overall exploratory activity of the animals.
- A quiet, dimly lit (10-15 lux) experimental room is the preferred environment for spontaneous exploratory behavior in mice.
- At the end of each day of the behavioral experiment, animals are all left undisturbed for one hour before they are transported back to the animal housing facility.
- All behavioral sessions are recorded using the video-tracking system. Whereas the object exploratory activity is a behavioral measure that could only be assessed with the objects present in a given context (during training and memory retention tests), general locomotor activity measures and animal tracking are performed during the pretraining session on Day 1 to assess group differences in locomotion and general exploratory activity, differences of which can bias assessment of novelty preference on the retention tests.
Acknowledgments
This behavioral protocol was originally used in Tsokas et al., 2016. This work was supported by NIH grants R21NS091830 and R01MH099128 (AAF) and R37MH057068, R01MH53576, R01DA034970, and the Lightfighter Trust (TCS).
References
- Barker, G. R. I. and Warburton, E. C. (2011). When is the hippocampus involved in recognition memory? J Neurosci 31(29): 10721-10731.
- Hardt, O., Migues, P. V., Hastings, M., Wong, J. and Nader, K. (2010). PKMζ maintains 1‐day‐and 6‐day‐old long‐term object location but not object identity memory in dorsal hippocampus. Hippocampus 20(6): 691-695.
- Kinnavane, L., Albasser, M. M. and Aggleton, J. P. (2015). Advances in the behavioural testing and network imaging of rodent recognition memory. Behav Brain Res 285: 67-78.
- Langston, R. F., Stevenson, C. H., Wilson, C. L., Saunders, I. and Wood, E. R. (2010). The role of hippocampal subregions in memory for stimulus associations. Behav Brain Res 215:275-291.
- Langston, R. F. and Wood, E. R. (2010). Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus 20(10): 1139-1153.
- Lee, A. M., Kanter, B. R., Wang, D., Lim, J. P., Zou, M. E., Qiu, C., McMahon, T., Dadgar, J., Fischbach-Weiss, S. C. and Messing, R. O. (2013). Prkcz null mice show normal learning and memory. Nature 493(7432): 416-419.
- Lee, I., Hunsaker, M. R. and Kesner, R. P. (2005). The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci 119:145-153.
- Lesburguères, E., Sparks, F. T., O’Reilly, K. C. and Fenton, A. A. (2016). Active place avoidance is no more stressful than unreinforced exploration of a familiar environment. Hippocampus 26(12): 1481-1485.
- Mumby, D. G., Gaskin, S., Glenn, M. J., Schramek, T. E. and Lehmann, H. (2002). Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem 9(2): 49-57.
- Norman, G. and Eacott, M. J. (2005). Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci 119:557-566.
- Steckler, T., Drinkenburg, W. H., Sahgal, A. and Aggleton, J. P. (1998a). Recognition memory in rats--I. Concepts and classification. Prog Neurobiol 54(3): 289-311.
- Steckler T, Drinkenburg, W. H., Sahgal, A. and Aggleton, J. P. (1998b). Recognition memory in rats--II. Neuroanatomical substrates. Prog Neurobiol 54:313-332.
- Tsokas, P., Hsieh, C., Yao, Y., Lesburgueres, E., Wallace, E. J., Tcherepanov, A., Jothianandan, D., Hartley, B. R., Pan, L., Rivard, B., Farese, R. V., Sajan, M. P., Bergold, P. J., Hernandez, A. I., Cottrell, J. E., Shouval, H. Z., Fenton, A. A. and Sacktor, T. C. (2016). Compensation for PKMζ in long-term potentiation and spatial long-term memory in mutant mice. Elife 5: e14846.
Article Information
Copyright
Lesburguères et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Lesburguères, E., Tsokas, P., Sacktor, T. C. and Fenton, A. A. (2017). The Object Context-place-location Paradigm for Testing Spatial Memory in Mice. Bio-protocol 7(8): e2231. DOI: 10.21769/BioProtoc.2231.
- Tsokas, P., Hsieh, C., Yao, Y., Lesburgueres, E., Wallace, E. J., Tcherepanov, A., Jothianandan, D., Hartley, B. R., Pan, L., Rivard, B., Farese, R. V., Sajan, M. P., Bergold, P. J., Hernandez, A. I., Cottrell, J. E., Shouval, H. Z., Fenton, A. A. and Sacktor, T. C. (2016). Compensation for PKMζ in long-term potentiation and spatial long-term memory in mutant mice. Elife 5: e14846.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











