- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measuring Behavioral Individuality in the Acoustic Startle Behavior in Zebrafish
(*contributed equally to this work) Published: Vol 7, Iss 7, Apr 5, 2017 DOI: 10.21769/BioProtoc.2200 Views: 10011
Reviewed by: Soyun KimZinan ZhouMasahiro Morita

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Behavioural Assay to Investigate Judgment Bias in Zebrafish
Felipe Espigares [...] Rui F. Oliveira
Feb 20, 2022 3275 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1832 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1481 Views
Abstract
The objective of this protocol is to provide a detailed description for the construction and use of a behavioral apparatus, the zBox, for high-throughput behavioral measurements in larval zebrafish (Danio rerio). The zBox is used to measure behavior in multiple individuals simultaneously. Individual fish are housed in wells of multi-well plates and receive acoustic/vibration stimuli with simultaneous recording of behavior. Automated analysis of behavioral movies is performed with MATLAB scripts. This protocol was adapted from two of our previously published papers (Levitz et al., 2013; Pantoja et al., 2016). The zBox provides an easy to setup flexible platform for behavioral experiments in zebrafish larvae.
Keywords: ZebrafishBackground
Behavioral differences between individuals in a population are ubiquitous and likely play a role in adaptation to varying selective pressures during evolution. However, variation in behavior among individuals is often ignored when the quantitation of the behavior of groups is presented as means and associated dispersions. In this protocol, we describe an approach to characterize individuality in the habituation of the acoustic startle behavior at the population level in zebrafish (Danio rerio) larvae. Our approach and set-up can be easily adapted to studies of individuality in other zebrafish larval behaviors.
Materials and Reagents
- Petri dishes
- Pipette tip
- 48-well multi-well plate
- Zebrafish embryos
- Instant ocean sea salt mix (Spectrum Brands, catalog number: SS15-10 )
- DI H2O
- E3 medium (see Recipes)
Equipment
- P1000 PIPETMAN pipettor
- Stir plate
- zBox parts list (Table 1)
Table 1. ZBox parts listManufacturer/Item description Part number Quantity Newegg - Computer Minimum requirements: PC, 8 GB RAM, 2 GHz processor 1 PCIe Firewire 800 card 9SIA24G28M5974 1 National Instruments (DAQ) NI PCI-6229 779068-01 1 RC68-68 Cable (1 m) 192061-01 2 CB-68LP - Unshielded 777145-01 2 McMaster Carr (Structural external) 80/20 1”x1” framing 96” 47065T123 4 80/20 corners 47065T177 20 80/20 nuts 80x 47065T147 12 Black PVC, 48”x48”x. 84775K942 1 236” (back)Black PVC 36”x48”x.236 84775K742 3 sides and top Super Adhesive Hook 94985K614 10ft Loop 9489K874 10ft Loctite Black Silicone sealant 74945A81 1 Black gaffer’s tape 7612A94 1 Right angle 80/20 47065T223 2 80/20 fastener 47065T139 2 Acrylic cement 7528A13 1 PTFE tape 4591K11 1 General Purpose Epoxy Permatex 84101 1 Nylon 6/6 Thumb Screw 96295A336 1 Thorlabs (Structural internal) Breadboard 24”x36” MB2436 1 XT66 Rail XT66-500 1 XT66 Dovetail XT66DP-500 1 XT66 Dovetail clamp 40 mm XT66C2 2 XT66 right angle cross piece XT66CB 1 XT66 base plate XT66P1 1 XT66 pivot platform XT66RC 2 Plate holder FP01 2 10” mounting post P10 1 Pedestal Base adapter PB4 1 Clamping fork PF175 1 Post mounting clamp C1501 1 Blackout fabric BK5 1 Right angle plate AP90 1 Edmund Optics 101 x 127 mm, 45 Degree AOI, Hot Mirror 43-958 2 Pike F-245 2/3” CCD camera 59-221 1 Pike Tripod adapter 59-227 Plastic IR filter 43-949 1 Navitar 50MM F/2.8 TELECENTRIC LENS TC-5028 1 Unibrain (1394store.com) 1m (15 ft) IEEE-1394b 9p to 9p screw lock cable 1 Digi-Key Electronics 100Kohm resistor CMF100KHFCT-ND 4 2-pin recipient A99613-ND 10 2-pin socket A99620-ND 20 2-pin header A30770-ND 10 Yellow LEDs C503B-AAN-CY0B0251-ND 48 IR LEDs 475-2919-ND 48 White LEDs C503C-WAS-CBADA151-ND 48 Blue LEDs C503B-BAN-CY0C0461-ND 48 1ohm resistor P1.0W-2BK-ND 3 270 ohm resistor PPC270W-1CT-ND 16 390 ohm resistor PPC390W-1CT-ND 6 Diode 1N914B-ND 2 12 V power supply EPS377-ND 1 24 V power supply EPS357-ND 1 LEDsupply BuckPuck DC LED Drivers 0302x-D-x-xxxx 1 Machine shop (plastics) Custom plastic fabrication 1 Whatman (GE) plates Multi-well plate of desired well number Visaton (speakers) 8006 2 Pyle Audio (amplifier) PyleHome PCA1 1 60 Watt incandescent bulb 1 Simple designs home (lamp) LD1003-WHT 1
Notes: Assembly of zBox (see Figures 1-7):- Use epoxy to adhere the two side walls into the grooves on the back panel (Figure 2).
- The screw holes in the LED platforms need to be drilled for 4-40 threads (Figure 3).
- Use the plastic thumbscrews to mount the 2 LED platforms (one for the imaging IR LEDs and one for LEDs for use in optogenetic experiments) to the slots in the side walls (Figure 4).
- LED arrays can be wired in 8 x 6 or 12 x 10 arrays depending on the desired amount of light. Due to perspective, the IR imaging LEDs are best arranged so that the arrays extend to the sides of the LED platform. Thus, given the distance from the plate, the IR LEDs can evenly light the extent of the multiwell plate.
- The BuckPack LED driver can be wired to power the LED arrays and be switched using TTL pulses from the DAQ
- The multiwell plate platform should be able to slide into the grooves on the side walls.
- Screw speakers to side of multiwell platform (Figures 4-6).
- Use diffuser paper to assure that IR light is evenly distributed throughout wells.
- Mount camera to P10 posts. XT66 rails should be constructed to hold IR mirrors (Figure 7).
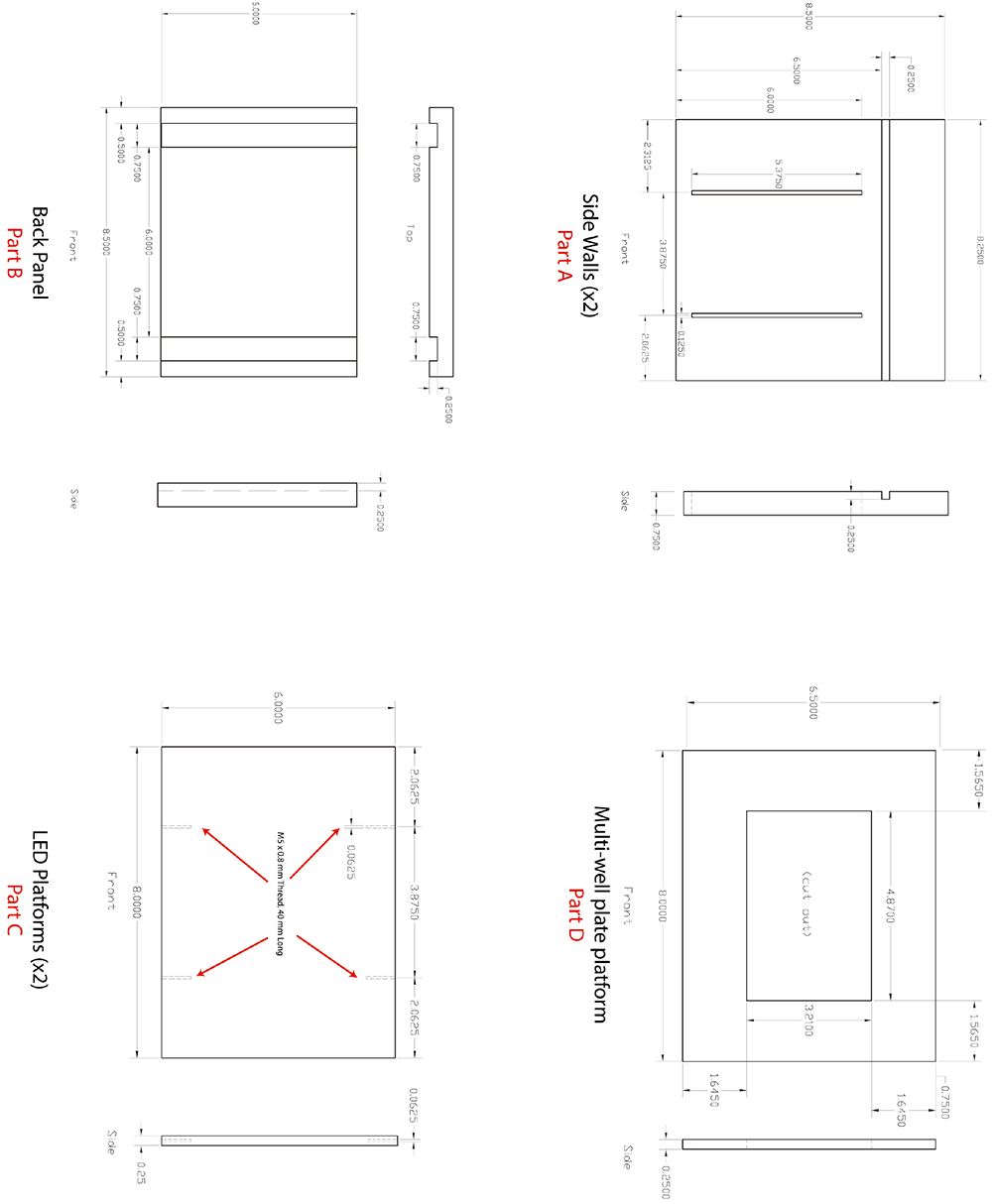
Figure 1. zBox plans
Figure 2. Slide side walls into back panel grooves and epoxy in place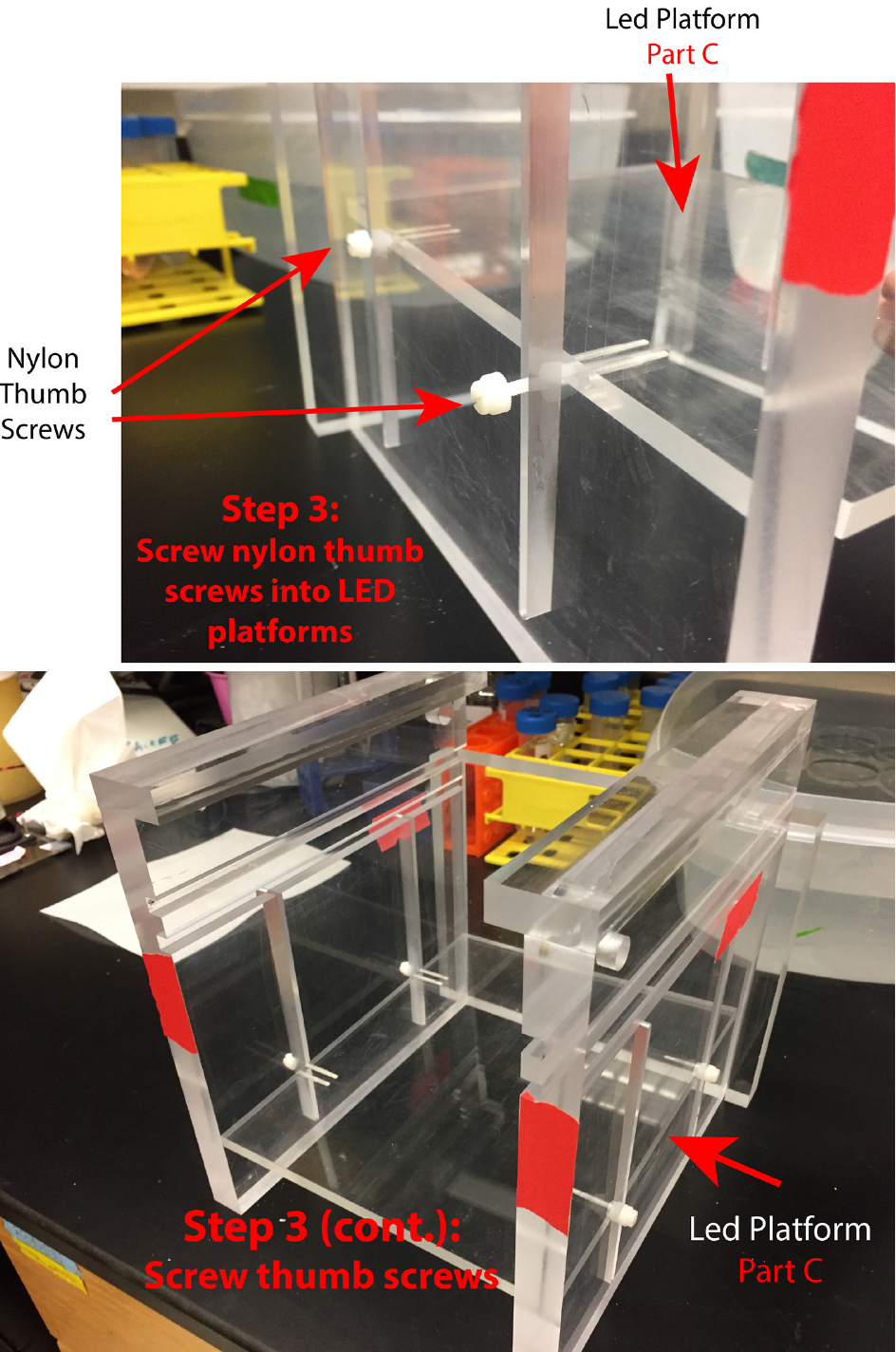
Figure 3. Use thumb screws to mount LED platforms into side wall slots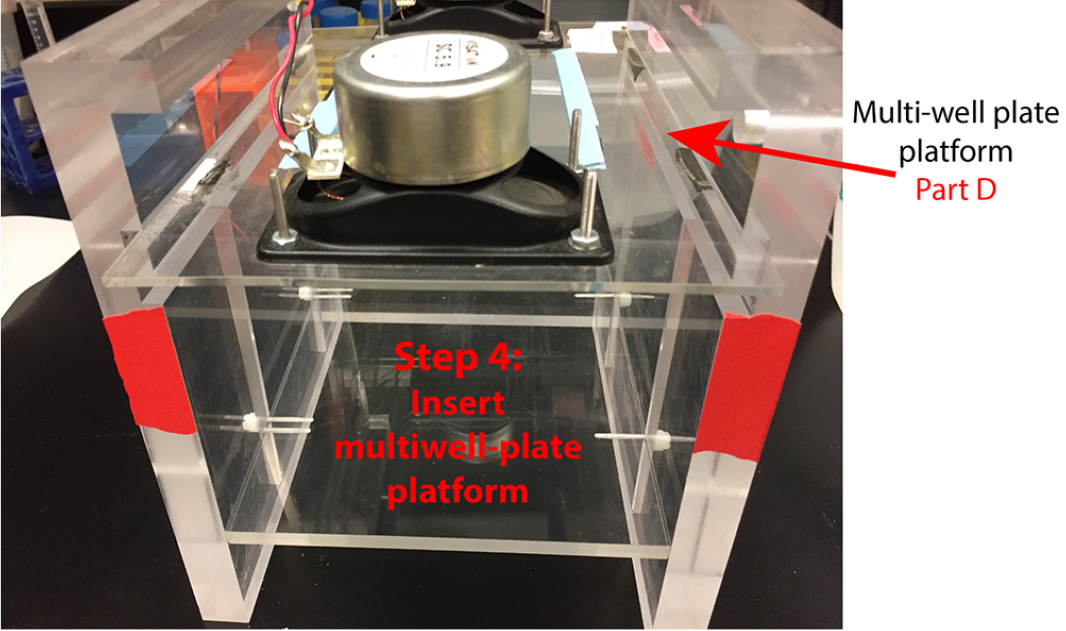
Figure 4. Insert multi-well plate platform into top grooves of side walls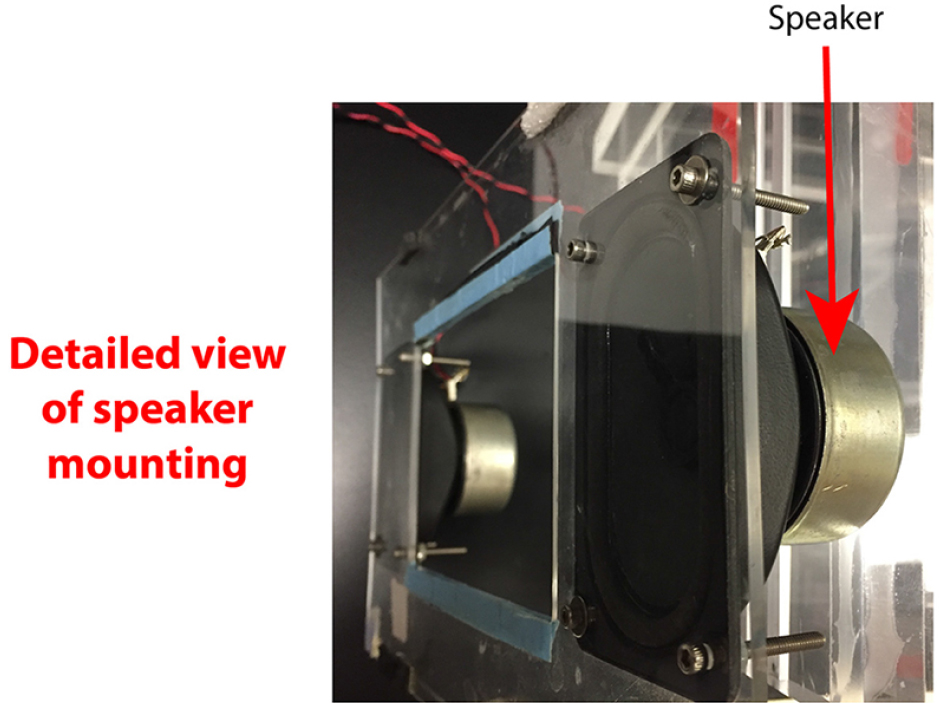
Figure 5. Detailed view of eaker mounting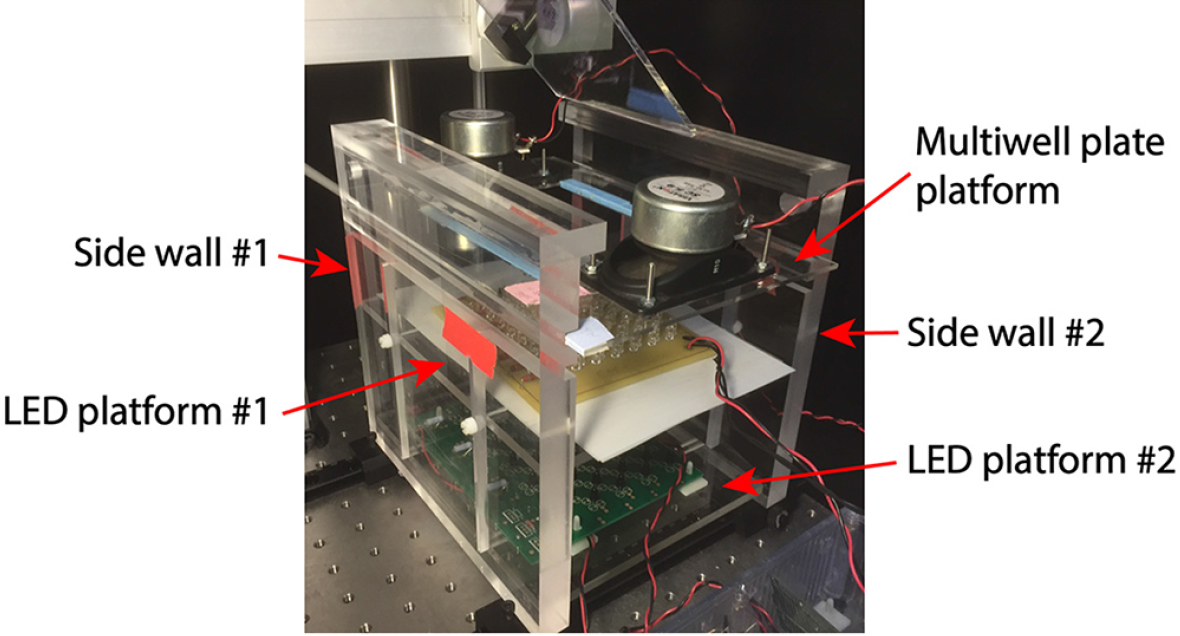
Figure 6. Completed zBox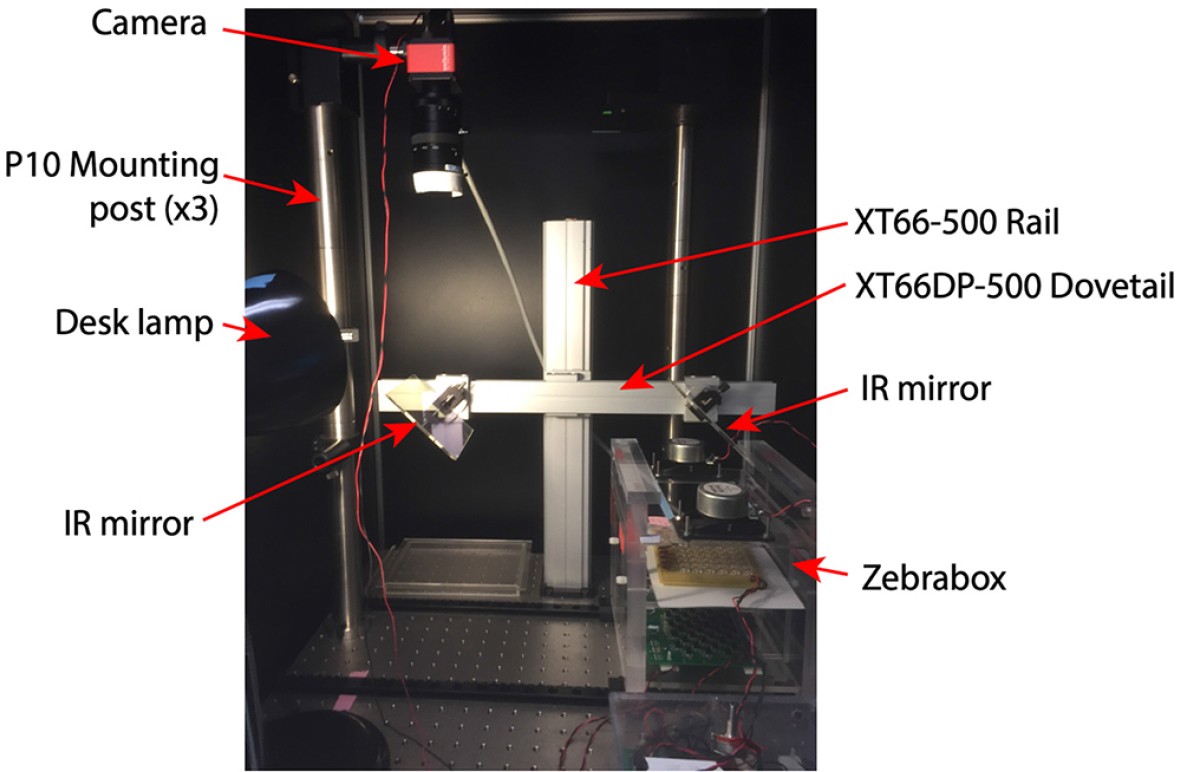
Figure 7. Camera mounting
- Use epoxy to adhere the two side walls into the grooves on the back panel (Figure 2).
Software
- Matlab2014a or later with Imaging Processing and Data Acquisition toolboxes installed
Procedure
- Zebrafish embryos are raised in Petri dishes at low densities (~2 fish per ml) at 28.5 °C in E3 medium.
- Larvae without inflated bladders and that do not respond to light taps on the dish on 4 dpf are removed.
- Larvae are individually pipetted in 300 µl of E3 medium with a P1000 PIPETMAN pipettor, with the pipette tip cut to prevent damage to the larvae, and individually transferred from Petri dishes to the wells of a 48-well multi-well plate.
- To prevent experimenter selection bias zebrafish larvae are randomly chosen for transfer to the 48-well plate.
- 48-well microplate is transferred to a plexiglass box (Figures 4-7) kept inside an isolated dark chamber. Interior of chamber illuminated with constant diffuse white light is supplied by a 60 W bulb.
- The behavioral apparatus is housed in a climate controlled environment at 22 °C.
- If exposed to different treatments, larvae from different groups are tested simultaneously in the same micro-well plate.
- Larvae used in single-day experiments are 6 dpf, whereas those used in the three-day long experiments are 5-7 dpf. For the three-day experiments, the larvae are kept in the microplates with 1,300 µl E3 overnight at 28.5 °C between measurements. The medium is refreshed the next day and then readjusted to 300 µl for the next habituation trial.
- Behavioral activity within the microplate is recorded with a CCD camera, which is backlit by an array of infrared LEDs.
- The behavioral assay consists of 15 min of acclimation to the high-throughput apparatus, followed by 10 min of spontaneous activity recording, and then the habituation protocol.
- Sound stimuli are administered by two speakers mounted to the same platform as the microplate. The speakers are powered by a 15 W amplifier and delivered 900 Hz square waves of ~3 msec duration. Stimuli are delivered via a Native Instruments PCI-6229 DAQ controlled by MATLAB.
- Habituation protocol consists of 10 stimuli at 90 sec ISI (0.4 V, ~95 dB) followed by 100 stimuli at 5 sec ISI (0.4 V, ~95 dB).
- For each behavior experiment, data is analyzed from randomly sampled larval zebrafish derived from multiple clutches obtained on different days. We empirically determined that measuring behavior in approximately 100 larvae is sufficient to quantify behavioral variation at the population level.
Data analysis
- Behavioral analysis is performed with MATLAB scripts (supplemental materials and Figure 8). README.txt file contains detailed information on how to run scripts. Basic skills in MATLAB programming are recommended.
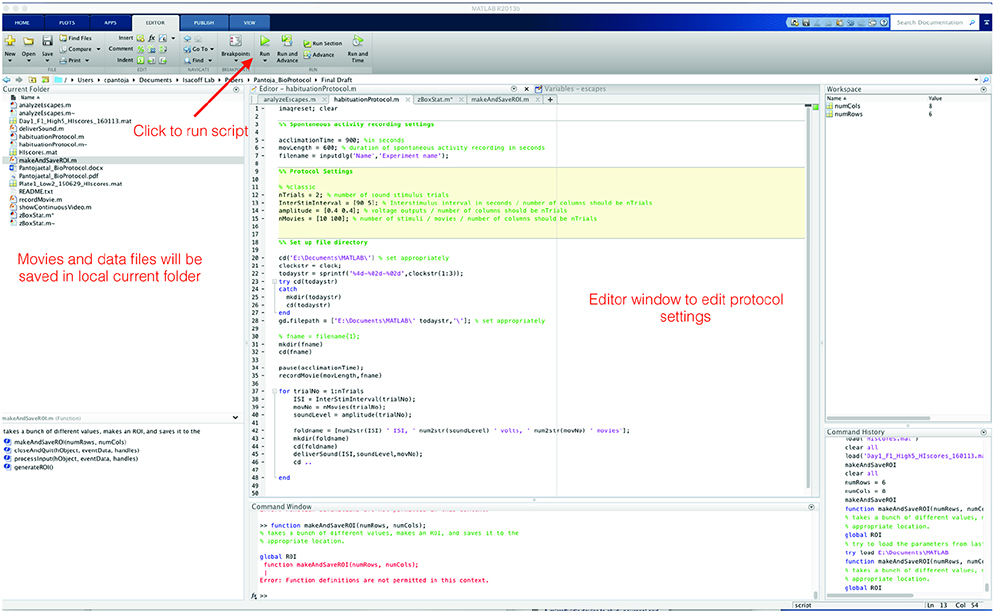
Figure 8. Screenshot of MATLAB user interface - Run habituationProtocol.m script. Escapes are detected using changes in pixel values between frames before and after sound stimulus presentation. An escape is counted if the difference of the integrated pixel values from the two frames immediately following the stimulus is statistically higher (P < 0.01, 2-sample t-test) than the distribution of pixel-change values taken from the non-escape portion spontaneous activity taken across all 120 movies (720 frames). The accuracy of this method was verified by visual inspection of the movies (Figure 9).
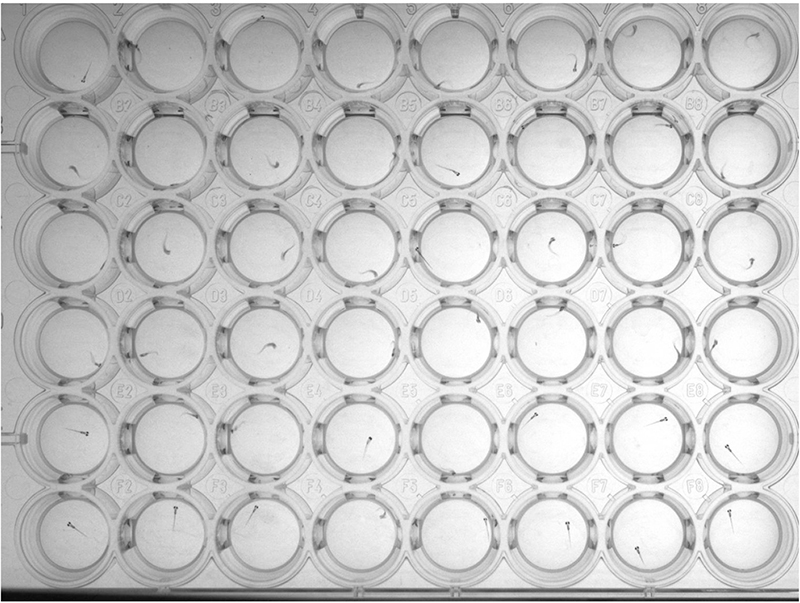
Figure 9. Representative image of 48-well plate during sound-induced escape - For analysis of behavioral movies run the analyzeEscapes.m script. We utilize the habituation index (HI) as a metric to describe acoustic startle habituation in individual larvae. The HI is defined as the difference in escape probability to the last 40 high-level stimuli under habituating conditions (10 sec inter-stimulus interval [ISI]) with the first 10 high-level stimuli under non-habituating conditions (90 sec ISI), divided by the probability of escape to the last 40 stimuli (HI =[Plast40 - Pfirst10]/Plast40). Only highly-responsive fish, probability of response 90% or more to the initial 10 high-level stimuli, are used in calculating the HI (Figure 10).
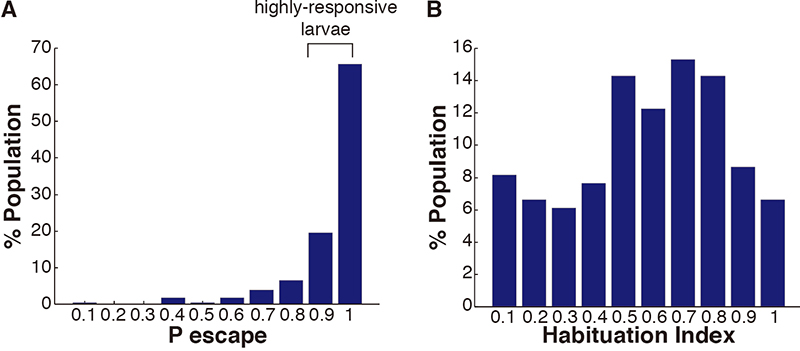
Figure 10. Graphic example of acoustic startle repose quantification. A. Frequency distribution of probability of escape values during the first 10 stimuli (ISI = 90 sec, n = 230) of habituation series. B. Frequency distribution of HIs for highly responsive fish in (A) (ISI = 10 sec, n = 196). - To determine if differences in HI between experimental groups are significant, we use the Mann-Whitney U-Test, which is included in the script zBoxStat.m. The HIscores.mat file in the supplemental materials provides an example of data to analyze.
Recipes
- E3 embryo medium
Note: It is not necessary to use filtered or sterile solutions. - Making 50x stock solution:
15 g instant ocean sea salt mix
1 L DI H2O
Mix on stir plate for ~5 min - Working solution:
200 ml 50x E3
10 L DI H2O
2 drops (~0.1 ml) of methylene blue
Acknowledgments
This work supported by the National Institutes of Health Nanomedicine Development Center for the Optical Control of Biological Function (PN2EY018241) and the Human Frontier Science Program (RGP0013/2010).
The zBox was initially developed by Dr. David Schoppik in Dr. Alex Schier’s laboratory. The work and experimental approach described here have been adapted from previous research published in Nature Neuroscience (Levitz et al., 2013) and Neuron (Pantoja et al., 2016). Further information about uses for the zBox are reported in a study that investigated the role of neuropeptides in the partition of arousal behaviors in zebrafish (Woods et al., 2014). In addition, work by Zhou et al. can be used as a complementary set of methods for the utilization of this apparatus (Zhou et al., 2014).
References
- Levitz, J., Pantoja, C., Gaub, B., Janovjak, H., Reiner, A., Hoagland, A., Schoppik, D., Kane, B., Stawski, P., Schier, A. F., Trauner, D. and Isacoff, E. Y. (2013). Optical control of metabotropic glutamate receptors. Nat Neurosci 16(4): 507-516.
- Pantoja, C., Hoagland, A., Carroll, E. C., Karalis, V., Conner, A. and Isacoff, E. Y. (2016). Neuromodulatory regulation of behavioral individuality in zebrafish. Neuron 91(3): 587-601.
- Woods, I. G., Schoppik, D., Shi, V. J., Zimmerman, S., Coleman, H. A., Greenwood, J., Soucy, E. R. and Schier, A. F. (2014). Neuropeptidergic signaling partitions arousal behaviors in zebrafish. J Neurosci 34(9): 3142-3160.
- Zhou, Y., Cattley, R. T., Cario, C. L., Bai, Q. and Burton, E. A. (2014). Quantification of larval zebrafish motor function in multiwell plates using open-source MATLAB applications. Nat Protoc 9(7): 1533-1548.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Pantoja, C., Hoagland, A., Carroll, E., Schoppik, D. and Isacoff, E. Y. (2017). Measuring Behavioral Individuality in the Acoustic Startle Behavior in Zebrafish. Bio-protocol 7(7): e2200. DOI: 10.21769/BioProtoc.2200.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








