- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Extraction, Purification and Quantification of Diffusible Signal Factor Family Quorum-sensing Signal Molecules in Xanthomonas oryzae pv. oryzae
Published: Vol 7, Iss 6, Mar 20, 2017 DOI: 10.21769/BioProtoc.2190 Views: 11444
Reviewed by: Zhaohui LiuSadri ZnaidiManuela Roggiani

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bacterial Infection and Hypersensitive Response Assays in Arabidopsis-Pseudomonas syringae Pathosystem
Minhang Yuan and Xiu-Fang Xin
Dec 20, 2021 5325 Views

Protocol for Inoculation of PGPR Staphylococcus sciuri to Seeds and Seedlings of Rice and Tomato Plants for Increased Root and Shoot Growth
Girija Somna [...] Dinakar Challabathula
Mar 20, 2025 2200 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1671 Views
Abstract
Bacteria use quorum-sensing (QS) systems to monitor and regulate their population density. Bacterial QS involves small molecules that act as signals for bacterial communication. Many Gram-negative bacterial pathogens use a class of widely conserved molecules, called diffusible signal factor (DSF) family QS signals. The measurement of DSF family signal molecules is essential for understanding DSF metabolic pathways, signaling networks, as well as regulatory roles. Here, we describe a method for the extraction of DSF family signal molecules from Xanthomonas oryzae pv. oryzae (Xoo) cell pellets and Xoo culture supernatant. We determined the levels of DSF family signals using ultra-performance liquid chromatographic system (UPLC) coupled with accurate mass time-of-flight mass spectrometer (TOF-MS). With the aid of UPLC/MS system, the detection limit of DSF was as low as 1 µM, which greatly improves the ability to detect DSF DSF family signal molecules in bacterial cultures and reaction mixtures.
Keywords: Quorum sensing (QS)Background
Xanthomonas oryzae pv. oryzae (Xoo) is a causal agent of bacterial blight disease of rice, and produces multiple DSF family QS signals, including cis-11-methy-dodecenoic acid (DSF), cis-2-dodecenoic acid (BDSF), cis-10-methyl-2-dodecenoic acid (IDSF) and cis,cis-11-methyldodeca-2,5-dienoic acid (CDSF), to regulate virulence factor production (Figure 1). The biosynthesis, perception, and turnover of DSF family signals require components of the rpf (regulation of pathogenicity factors) cluster in Xoo. RpfF is a key DSF biosynthase with both acyl-ACP thioesterase and dehydratase activity. The two-component system, comprising the sensor kinase RpfC and the response regulator RpfG, plays an essential role in the perception and transduction of DSF family signals. RpfB has recently been characterized as a fatty acyl-CoA ligase (FCL), which functions in DSF family signal turnover in Xanthomonas (Wang et al., 2016; Zhou et al., 2015b). Deletion of rpfB in Xoo strain PXO99A leads to an over-production of DSF and BDSF and reduced production of extracellular polysaccharide (EPS), extracellular amylase activity. Moreover, attenuated pathogenicity has also been observed (Wang et al., 2016). Therefore, the RpfB-dependent DSF family signal turnover system is considered a naturally occurring signal turnover system in Xanthomonas. Detection and quantification of DSF family signals are very important in understanding the mechanisms of the DSF signaling system. As a result, detection methods for these signals have improved over the past few years. Initially, DSF detection relied on genetically engineered DSF biosensor-based detection systems (Slater et al., 2000; Wang et al., 2004), which provide an indirect way to analyze the activity of DSF family signals without differentiating structurally similar members of this group. Later, a detection method based on high-performance liquid chromatography (HPLC) was developed, which allowed a direct quantification of production levels of DSF family signal molecules by Xanthomonas (Wang et al., 2004; He et al., 2010; Zhou et al., 2015a). Recently, this HPLC-based method was further improved by using ultra performance liquid chromatographic system/ mass spectrometry (UPLC/MS),which offers better sensitivity and accuracy in the measurement of DSF family signals produced by Xoo, which will be presented in detail in this protocol (Zhou et al., 2015b; Wang et al., 2016).
Figure 1. Chromatogram of ethyl acetate extract of the culture supernatant of the DSF hyper-production mutant ΔrpfCΔrpfB of Xoo strain PXO99A. A. Four molecules of the DSF family QS signals are detected in the supernatant of ΔrpfCΔrpfB in nutrient broth. Among them, DSF and BDSF are the predominant signal molecules. B. The chemical structure of the four DSF family signal molecules.
Materials and Reagents
- Pipette tips
2-100 μl (Eppendorf, catalog number: 0030000.870 )
50-1,000 μl (Eppendorf, catalog number: 0030000.919 )
1-10 ml (Eppendorf, catalog number: 0030000.765 ) - 2.0 ml microtubes (Corning, Axygen®, catalog number: MCT-200-C )
- pH test strips (0.0-6.0 pH) (Sigma-Aldrich, catalog number: P4661 )
- 1.5 ml microtubes (Corning, Axygen®, catalog number: MCT-150-C )
- 50 ml centrifuge tube (Corning, catalog number: 430829 )
- Acrodisc® MS syringe filters (0.2 µm, 13 mm, WWPTFE membrane) (Pall, catalog number: MS-3301 )
- BD Tuberculin syringe with detachable needle (1 ml, 27 G x 1/2 in.) (BD, catalog number: 309623 )
- 1.5 ml Semi-micro cuvette (AS ONE, catalog number: 1-2855-02 )
- HPLC screw cap vials (Agilent Technologies, catalog number: 5182-0714 )
- 400 µl polypropylene flat bottom insert (Agilent Technologies, catalog number: 5183-2087 )
- Zorbax Eclipse XDB-C18 reverse phase column (Analytical, 4.6 x 150 mm, 5-micron) (Agilent Technologies, catalog number: 993967-902 )
- Sterile Petri dishes (90 mm) (Sartorius, catalog number: 14-555-735 )
- Xoo strain ΔrpfB, the rpfB deletion mutant of Xoo strain PXO99A, which overproduces DSF family signal molecules (Wang et al., 2016)
- Cephalexin (Sigma-Aldrich, catalog number: 1099008 )
- DSF (cis-11-methy-dodecenoic acid) (HPLC grade, purity ≥ 90.0%) (Sigma-Aldrich, catalog number: 42052 )
- BDSF (cis-2-dodecenoic acid) (HPLC grade, purity ≥ 90.0%) (Sigma-Aldrich, catalog number: 49619 )
- 6 N hydrochloric acid solution (HCl) (Sigma-Aldrich, catalog number: 13-1686 )
- Ethyl acetate (ACS reagent grade, purity ≥ 99.5%) (Sigma-Aldrich, catalog number: 676810 )
- Methanol (HPLC grade) (Fisher Scientific, catalog number: A452-4 )
- Phosphate buffered saline (PBS, pH 7.4) (Sigma-Aldrich, catalog number: P5368 )
- Glycerol (Sigma-Aldrich, catalog number: 49781 )
- Bacto peptone (BD, BactoTM, catalog number: 211677 )
- Bacto beef extract (BD, BactoTM, catalog number: 211520 )
- Sucrose (VetecTM reagent grade) (Sigma-Aldrich, catalog number: V900116 )
- BBL yeast extract (BD, BBL, catalog number: 211931 )
- NaOH
- Agar (BD, catalog number: 281230 )
- Sodium acetate (ACS reagent grade, purity ≥ 99.0%) (Sigma-Aldrich, catalog number: 791741 )
- Acetic acid (ACS reagent grade, purity ≥ 99.7%) (Sigma-Aldrich, catalog number: 695092 )
- Sterile deionized H2O
- Glycerol stock (see Recipes)
- Nutrient broth (NB, see Recipes)
- Nutrient agar (NA, see Recipes)
- 0.2 M sodium acetate solution (pH 8.0) (see Recipes)
- 0.2 M acetic acid solution (pH 2.7) (see Recipes)
- 0.2 M sodium acetate buffer (pH 3.8) (see Recipes)
- Cephalexin stock solution (20 mg/ml, see Recipes)
Equipment
- Corning® glass Erlenmeyer flasks with screw cap
50 ml (Corning, catalog number: 4985-50 )
250 ml (Corning, catalog number: 4985-250 ) - MaxQTM 6000 Incubated/Refrigerated shaker (Thermo Fisher Scientific, Thermo ScientificTM, model: MaxQTM 6000 , catalog number: SHKE6000-8CE)
- UV-visible spectrophotometer (Thermo Fisher Scientific, Thermo ScientificTM, model: BioMateTM 3S )
- Microcentrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: SorvallTM LegendTM Micro 17R )
- Centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM MultifugeTM X1R )
- Vortex mixer (VWR, catalog number: 10153-840 )
- CentriVap benchtop concentrator with glass lid (Labconco, catalog number: 7810040 )
- Pipettes
10-100 μl (Eppendorf, catalog number: 3120000046 )
100-1,000 μl (Eppendorf, catalog number: 3120000062 )
1-10 ml (Eppendorf, catalog number: 3120000089 ) - Fume hood
- Refrigerator (MEILING BIOLOGY & MEDICAL, model: DW-YL270 )
- VibracellTM High Intensity Ultrasonic Liquid Processors (Sonics & Materials, model: VCX 500 ) connected with a Tapered Microtip probe (tip diameter: 3 mm) (Sonics & Materials, catalog number: 630-0422 )
- Digital precise water bath (DAIHAN Scientific, model: WB-6 )
- Pear shaped glass flask, Ts 29/38, 100 ml (Tokyo Rikakikai, EYELA, catalog number: 116150 )
- UPLC/MS system
Ultra-performance liquid chromatographic system (UPLC) (Agilent Technologies, model: Agilent 1290 Infinity LC ) coupled with an accurate mass time-of-flight (TOF) MS (Agilent Technologies, model: Agilent 6230 Accurate-Mass TOF MS ) equipped with an Agilent Jet Stream (AJS) electrospray ionization (ESI) source - Diode array detector (Agilent Technologies, model: G4212A )
- pH meter (Mettler Toledo, model: FE20 )
- Diaphragm vacuum pump (Labconco, catalog number: 7393001 )
- Rotary evaporator (Tokyo Rikakikai, EYELA, model: N-1100 )
- Circulation cooling-water system (Tokyo Rikakikai, EYELA, model: CCA-1111 )
- Autoclave (Panasonic Healthcare, model: MLS-3781L )
Software
- Agilent MassHunter Workstation Data Acquisition Software (revision B.04)
Procedure
- Preparation of the pre-culture (to be used for all subsequent culture conditions)
- Streak Xoo strain ΔrpfB from -80 °C glycerol stock on NA plate supplemented with cephalexin at a final concentration of 20 µg/ml.
- Incubate the plate at 28 °C for 4 days to obtain single colonies.
- With a pipette tip, isolate one ΔrpfB colony and inoculate in 10 ml of NB supplemented with cephalexin with a final concentration of 20 µg/ml in a 50 ml Erlenmeyer flask.
- Incubate the ΔrpfB culture in the MaxQTM 6000 Incubated/Refrigerated shaker at 28 °C with shaking at 200 rpm for 36 h.
- Streak Xoo strain ΔrpfB from -80 °C glycerol stock on NA plate supplemented with cephalexin at a final concentration of 20 µg/ml.
- Preparation of Xoo culture
- Measure the optical density at 600 nm (OD600) of the 1:4 diluted ΔrpfB pre-culture in a spectrophotometer and calculate the optical density of the pre-culture by multiplying the measured reading by 4 (the dilution ratio).
- Adjust the OD600 of the pre-culture to approximately 1.0 using NB.
- Add 1 ml of adjusted ΔrpfB pre-culture to 50 ml nutrient broth (1: 50 dilution) in a 250 ml Erlenmeyer flask with vent cap.
- Incubate the culture at 28 °C with shaking at 200 rpm for 36-48 h until it reaches early stationary phase (OD600 ≈ 2.8-3.0) for DSF/BDSF extraction.
Note: If other bacterial strains are assayed, other specific growth conditions should be optimized.
- Measure the optical density at 600 nm (OD600) of the 1:4 diluted ΔrpfB pre-culture in a spectrophotometer and calculate the optical density of the pre-culture by multiplying the measured reading by 4 (the dilution ratio).
- Extracellular DSF/BDSF extraction
- Dispense 4 ml of the ΔrpfB culture in two 2 ml centrifuge tubes and centrifuge at 8,000 x g for 15 min to obtain the culture supernatant for extracellular DSF/BDSF extraction.
- Transfer the supernatant to two new 2 ml microtubes and adjust its pH to 3.0-3.5 by adding adequate volume (usually 15 to 20 µl) of 6 N hydrochloric acid and monitoring pH changes with test strips.
- Dispense the supernatant in eight 2 ml microtubes (0.5 ml per tube), and then, add 1 ml of ethyl acetate into each tube.
- Vortex the microtubes at the highest speed for 5 min to extract DSF and BDSF molecules and centrifuge at 8,000 x g for 10 min to separate the ethyl acetate fraction from the aqueous fraction.
- Carefully collect the ethyl acetate fractions (upper layer) into four 1.5 ml microtubes by pipetting and evaporate the solvent in a CentriVap benchtop concentrator at 40 °C to complete dryness (approximately 20 min).
- Dispense 0.15 ml of methanol in the four 1.5 ml microtubes and vortex the microtubes vigorously for 30 sec, which ensures that all residues are re-dissolved.
- Centrifuge the microtubes at 2,000 x g for 1 min, and then transfer the extraction solution from each microtube into a new 1.5 ml microtube using a pipette.
- Evaporate the solvent in the CentriVap benchtop concentrator at 40 °C to complete dryness (approximately 40 min).
Notes:- At this point, the samples may be frozen at -20 °C for later steps or analyzed immediately as described in the following steps.
- For protection from inhalation of volatile solvents, steps C3 to C8 in this section should be performed in a fume hood.
- At this point, the samples may be frozen at -20 °C for later steps or analyzed immediately as described in the following steps.
- Dispense 4 ml of the ΔrpfB culture in two 2 ml centrifuge tubes and centrifuge at 8,000 x g for 15 min to obtain the culture supernatant for extracellular DSF/BDSF extraction.
- Intracellular DSF/BDSF extraction
- Decant 40 ml ΔrpfB culture in a 50 ml centrifuge tubes and harvest bacterial cells by centrifuging at 8,000 x g for 15 min at 4 °C.
- Discard the supernatant and add 40 ml of 1x PBS buffer in the tube to wash the cell pellets by pipetting gently.
- Discard the supernatant and harvest the washed bacterial cells by centrifuging at 8,000 x g for 15 min at 4 °C.
- Re-suspend the cell pellets in 10 ml of ice-cold sodium acetate buffer (pH 3.8).
- Freeze the re-suspension solution in a -20 °C freezer for 1 h and then incubate the bacterial suspension at room temperature for 30 to 60 min to thaw the suspension completely.
- Homogenize the cells by pipetting the thawed bacterial suspension gently.
- Repeat steps D5 and D6 once more.
- Sonicate the cell homogenate for a total of 6 min by sonicating for 3 sec, pausing for 4 sec, and repeating this sonication/pause cycle120 times (amplitude set at 20%) on ice using the Vibra cellTM High Intensity Ultrasonic Liquid Processors connected with a Tapered Microtip probe (3 mm diameter).
- Transfer the centrifuge tube containing the processed cell lysate into a water bath for 5 min at 95 °C to denature the total protein.
- Separate the soluble lysis solution with bacterial cell debris by centrifuging at 8,000 x g for 15 min at 4 °C.
- After centrifugation, immediately transfer the soluble lysis solution (the transparent part) to a 250 ml Erlenmeyer glass flask using a pipette. This should be performed carefully to avoid disturbing the precipitate at the bottom of the tube.
- Add 20 ml of ethyl acetate in the flask and close the flask with the cap immediately to avoid evaporation and spillage of the volatile solvent. Incubate the capped flask in the MaxQTM 6000 shaker at 28 °C with shaking at 200 rpm for 10 min to extract DSF and BDSF.
- Transfer the extraction mixture to a 50 ml centrifuge tube and centrifuge at 6,000 x g for 10 min to separate the ethyl acetate fraction from the aqueous fraction.
- Transfer the ethyl acetate fractions (upper layer) to a 100 ml pear shaped glass flask by pipetting carefully.
- Remove the solvent by rotary evaporation at 40 °C to complete dryness (approximately 5 min).
- Dispense 1 ml of methanol into the pear shaped glass flask to re-dissolve the residue by pipetting several times.
- Transfer the solution to a 1.5 ml microtube and evaporate the solvent in the CentriVap benchtop concentrator at 40 °C to complete dryness.
Notes:- At this point, the samples may be frozen at -20 °C for later steps or be analyzed immediately as described in the following steps.
- For protection from inhalation of volatile solvents, steps D12 to D17 in this section should be performed in a fume hood.
- At this point, the samples may be frozen at -20 °C for later steps or be analyzed immediately as described in the following steps.
- Decant 40 ml ΔrpfB culture in a 50 ml centrifuge tubes and harvest bacterial cells by centrifuging at 8,000 x g for 15 min at 4 °C.
- LC/MS sample preparation
- Crude extraction samples can be obtained by adding 200 µl of methanol into the 1.5 ml microtube and vortex at the highest speed.
- To remove any insoluble particles in the sample, filter the crude sample through a MS syringe filter (0.2 µm) connected with an 1 ml disposable syringe. Collect the filtered sample (approximately 100 µl) in a new 1.5 ml microtube.
- Transfer 60 µl of filtered sample into a chromatography vial fitted with a flat bottom insert using a pipette. Cap the vials. Samples are now ready for LC/MS analysis.
- Crude extraction samples can be obtained by adding 200 µl of methanol into the 1.5 ml microtube and vortex at the highest speed.
- LC/MS procedure
- Use HPLC grade methanol (eluent B)-water (eluent A) (80:20, v/v) as mobile phase.
- Maintain the column temperature at 30 °C and flow rate at 0.4 ml/min.
- Equilibrate the column for 15 min or longer until the baseline is stable.
- Inject 5 μl aliquots of prepared samples onto an Agilent 1290 Infinity UPLC with a Zorbax Eclipse XDB-C18 column.
- Detect DSF and BDSF molecules using a diode array detector (Agilent G4212A) set to a detection wavelength of 220 nm with a band width of 4 nm.
- The sample is then injected into an AJS ESI ion-trap mass spectrometer in negative ionization mode.
- MS source parameters are as follows:
- Gas temperature: 325 °C
- Drying gas: 8 L min-1
- Nebulizer: 35 psig
- Sheath gas temperature: 350 °C
- Sheath gas flow: 11 L min-1
- Capillary voltage (Vcap): 3,500 V
- Nozzle voltage: 200 V
- The mass range: m/z 100-1,700
- Use HPLC grade methanol (eluent B)-water (eluent A) (80:20, v/v) as mobile phase.
Data analysis
- Use the Agilent MassHunter Workstation Data Acquisition Software (revision B.04) to acquire data in centroid mode. The single mass-to-charge (m/z) expansion for the chromatogram is symmetric 20.0 ppm. Typical mass spectra are shown in Figures 2-4.
- For [BDSF-H]- detection, set the monoisotopic exact value (m/z) to 197.1547 (Figure 2)
- For [CDSF-H]- detection, set the monoisotopic exact value (m/z) to 209.1547 (Figure 3).
- For [DSF-H]- and [IDSF-H]- detection, set the monoisotopic exact value (m/z) to 211.1704 (Figure 4).
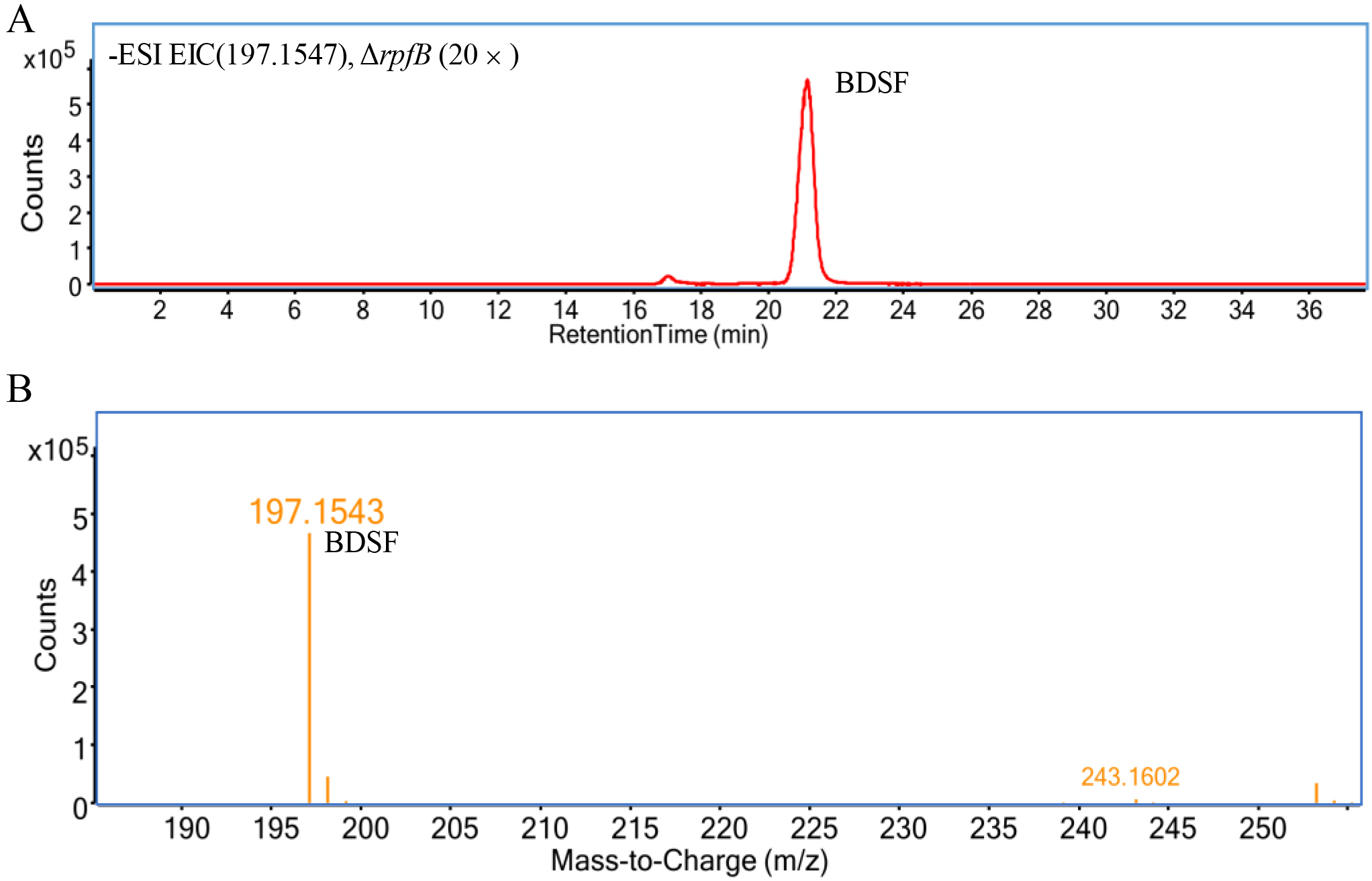
Figure 2. Typical mass spectrum of BDSF. A. Extracted ion chromatogram (counts vs. acquisition time) of BDSF in the ethyl acetate extract of the culture supernatant of ΔrpfB dissolved in methanol (20 times concentrated), in which the y-axis indicates the counts (absolute abundance) of ionized molecules and the x-axis indicates retention time. B. MS analysis of BDSF (counts vs. mass-to-charge [m/z]) shows an exact molecular weight (z = 1) of 197.1543 Da for [BDSF-H]- (the major peak), which determines that the exact molecular weight of BDSF is 198.1547. The y-axis indicates the counts (absolute abundance) of ionized molecules, and the x-axis indicates mass-to-charge (m/z) of ionized molecules acquired from the BDSF peak in the upper panel (Panel A).
Figure 3. Typical mass spectrum of CDSF. A. Extracted ion chromatogram (counts vs. acquisition time) of CDSF in the ethyl acetate extract of the culture supernatant of ΔrpfB dissolved in methanol (20 times concentrated), in which the y-axis indicates the counts (absolute abundance) of ionized molecules and the x-axis indicates retention time. B. MS analysis of CDSF (counts vs. mass-to-charge [m/z]) shows an exact molecular weight (z = 1) of 209.1547 Da for [CDSF-H]- (the major peak), which determines that the exact molecular weight of CDSF is 210.1547. The y-axis indicates the counts (absolute abundance) of ionized molecules, and the x-axis indicates mass-to-charge (m/z) of ionized molecules acquired from the CDSF peak in the upper panel (Panel A).
Figure 4. Typical mass spectrum of DSF and IDSF. A. Extracted ion chromatogram (counts vs. acquisition time) of DSF and IDSF in the ethyl acetate extract of the culture supernatant of ΔrpfB dissolved in methanol (20 times concentrated), in which the y-axis indicates the counts (absolute abundance) of ionized molecules and the x-axis indicates retention time. B. MS analysis of DSF (counts vs. mass-to-charge [m/z]) shows an exact molecular weight (z = 1) of 211.1700 Da for [DSF-H]- (the major peak), which determines that the exact molecular weight of DSF is 212.1704. The y-axis indicates the counts (absolute abundance) of ionized molecules, and x-axis indicates mass-to-charge (m/z) of ionized molecules acquired from the DSF peak in the top panel (Panel A). C. MS analysis of IDSF (counts vs. mass-to-charge [m/z]) shows an exact molecular weight (z = 1) of 211.1704 Da for [IDSF-H]- (the major peak), which determines that the exact molecular weight of IDSF is 212.1704. The y-axis indicates the counts (absolute abundance) of ionized molecules, and the x-axis indicates mass-to-charge (m/z) of ionized molecules acquired from the IDSF peak in the top panel (Panel A). - Integrate and quantify peak areas.
- Create a standard curve for DSF or BDSF by plotting the peak area vs. the known concentrations. Example standard curves are shown in Figure 5, in which the DSF or BDSF standards at the concentrations of 1 µM, 5 µM, 10 µM and 50 µM were used (Zhou et al., 2015b).
Note: Since IDSF and CDSF are not commercially available, the standard curve for either of these two signal molecules was not created in the previous studies.
Figure 5. Example standard curves constructed by measuring the peak intensity (PI) of varying concentrations of BDSF (A) and DSF (B) (Zhou et al., 2015b) - Use the slope and y-intercept from the standard curve to calculate the concentration of DSF or BDSF in the samples. Data can be further normalized to cell count to determine the amount of DSF and BDSF per cell if necessary.
Notes
- This protocol is optimized for measuring DSF family signal levels in Xoo. For other bacteria, use the appropriate growth medium, antibiotics, and growth conditions.
- The production of DSF family QS signals in Xoo is growth phase-dependent. The rpfB gene of Xanthomonas is involved in DSF family signal turnover in vivo in the late stationary phase (Wang et al., 2016; Zhou et al., 2015b). The highest levels of DSF family signals produced by Xoo strains containing functional RpfB are present only in the early stationary phase and decrease rapidly thereafter; while the rpfB deletion mutant accumulates DSF family signals during growth (Wang et al., 2016). As a result, in order to measure DSF family signals in Xoo cells, it is essential to collect Xoo samples during the appropriate growth stage (early stationary phase), especially for those strains with functional RpfB.
- As mass spectrometry is a very sensitive technique, a DSF/BDSF deficient strain is recommended as a negative control for DSF/BDSF extraction and quantification in each individual experiment. For example, the DSF/BDSF deficient mutant ΔrpfF of Xoo could be used as a control sample.
- Acidification before extraction is a critical step for DSF/BDSF extraction in this protocol. Ensure the culture supernatant is acidified (pH less than 4.0).
Recipes
- Glycerol stock for one vial
150 µl 87% glycerol
500 µl Xoo overnight culture - Nutrient broth (1 L)
5 g Bacto peptone
3 g Bacto beef extract
10 g sucrose
1 g BBL yeast extract
Bring the volume to 1 L with dH2O and adjust the pH to 6.8 with NaOH
Sterilize the medium by autoclaving for 20 min at 115 °C - Nutrient agar (200 ml)
Add 3 g of agar to 200 ml of nutrient broth
Sterilize the medium by autoclaving for 20 min at 115 °C - 0.2 M sodium acetate solution, pH 8.0 (100 ml)
Dissolve 1.64 g sodium acetate in 100 ml dH2O - 0.2 M acetic acid solution, pH 2.7 (100 ml)
Dispense 1.15 ml acetic acid in 98.85 ml dH2O - 0.2 M sodium acetate buffer, pH 3.8 (100 ml)
12 ml 0.2 M sodium acetate solution
88 ml 0.2 M acetic acid solution - Cephalexin stock solution (1 ml)
Dissolve 20 mg cephalexin powder in 1 ml of sterile dH2O
Sterilize by filtration
Acknowledgments
This protocol is adapted from Zhou et al. (2015b) and Wang et al. (2016).
This work was supported by the research grants from the National Key Research and Development Program of China (No. 2016YFE0101000 to ZL) and the National Natural Science Foundation of China (No. 31471743 to HYW, No. 31301634 to ZL).
References
- He, Y. W., Wu, J., Cha, J. S. and Zhang, L. H. (2010). Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol 10: 187.
- Ryan, R. P., An, S. Q., Allan, J. H., McCarthy, Y. and Dow, J. M. (2015). The DSF family of cell-cell signals: an expanding class of bacterial virulence regulators. PLoS Pathog 11(7): e1004986.
- Slater, H., Alvarez-Morales, A., Barber, C. E., Daniels, M. J. and Dow, J. M. (2000). A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol 38(5): 986-1003.
- Wang, L. H., He, Y., Gao, Y., Wu, J. E., Dong, Y. H., He, C., Wang, S. X., Weng, L. X., Xu, J. L., Tay, L., Fang, R. X. and Zhang, L. H. (2004). A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51(3): 903-912.
- Wang, X. Y., Zhou, L., Yang, J., Ji, G. H. and He, Y. W. (2016). The RpfB-dependent quorum sensing signal turnover system is required for adaptation and virulence in rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 29(3): 220-230.
- Zhou, L., Wang, X. Y., Sun, S., Yang, L. C., Jiang, B. L. and He, Y. W. (2015a). Identification and characterization of naturally occurring DSF-family quorum sensing signal turnover system in the phytopathogen Xanthomonas. Environ Microbiol 17(11): 4646-4658.
- Zhou, L., Yu, Y., Chen, X., Diab, A. A., Ruan, L., He, J., Wang, H. and He, Y. W. (2015b). The multiple DSF-family QS signals are synthesized from carbohydrate and branched-chain amino acids via the FAS elongation cycle. Sci Rep 5: 13294.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhou, L., Wang, X., Zhang, W., Sun, S. and He, Y. (2017). Extraction, Purification and Quantification of Diffusible Signal Factor Family Quorum-sensing Signal Molecules in Xanthomonas oryzae pv. oryzae. Bio-protocol 7(6): e2190. DOI: 10.21769/BioProtoc.2190.
Category
Microbiology > Microbial biofilm > Quorum sensing factor
Plant Science > Plant immunity > Host-microbe interactions
Biochemistry > Other compound > Acid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











