- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Primary Culture of Adult Human Adipose-derived Stromal/Stem Cells
Published: Vol 7, Iss 5, Mar 5, 2017 DOI: 10.21769/BioProtoc.2161 Views: 14657
Reviewed by: Federica PisanoNingfei AnAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
Adipose-derived stromal/stem cells (ASCs) are multipotent cells that can be isolated from adipose tissue. Studies have shown that cells have the capacity to self-renew and differentiate into adipocyte, chondrocyte, myocyte, and osteoblast lineages. Thus, significant interest regarding their use for regenerative purposes to restore aging or damaged tissue has grown in recent decades. These cells have also been shown to immunomodulate the microenvironment and secrete abundant growth factors, which minimize inflammation and aid repair and regeneration. ASCs can be readily isolated from the stromal vascular fraction (SVF) of lipoaspirates. Given their ease of accessibility, bountiful source, and potential application in regenerative medicine and tissue engineering, there is growing interest in the characterization and utilization of ASCs. This protocol describes the isolation of ASCs from adult human adipose tissue as well as methods for culture maintenance including expansion and cryopreservation.
Keywords: Adipose-derived stem cellsBackground
Adipose-derived stromal/stem cells (ASCs) demonstrate vast potential for the field of stem cells. Following the therapeutic marvel of hematopoietic stem cells transplantation, ASCs represent the future for stem cells due to their more freely accessible source – adipose tissue. The ability of ASCs to self-renew and differentiate into various tissue lineages including adipocyte, chondrocyte, myocyte, and osteoblast lineages, allows restoration of damaged tissue. Additionally, it is speculated that ASCs have the potential to replicate tissue in vitro. In vitro organs will allow more readily available assessment of novel pharmaceuticals and thus reduce drug production costs significantly. However, inconsistencies in the processes of isolation, maintenance, and cryopreservation, prohibit collective analysis of results from different laboratories worldwide. A standard protocol for isolation and culture of ASCs is necessary to ensure consistent data analysis.
Materials and Reagents
- Stericup-GP 0.22 µm polyethersulfone 500 ml radio-sterilized vacuum filtration flask (EMD Millipore, catalog number: SCGPU05RE )
- Centrifuge tubes:
15 ml (Corning, catalog number: 430790 )
50 ml (Corning, catalog number: 430828 ) - Sterile disposable serologic pipets:
2 ml (Corning, Falcon®, catalog number: 357558 )
5 ml (Corning, Falcon®, catalog number: 357543 )
10 ml (Corning, Falcon®, catalog number: 357551 )
25 ml (Corning, Falcon®, catalog number: 357525 )
50 ml (Corning, Falcon®, catalog number: 357550 ) - NuncTM 15 cm diameter, 145 cm2 culture area cell culture/Petri dishes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 168381 )
- FisherbrandTM Premium microcentrifuge tubes: 1.5 ml (Fisher Scientific, catalog number: 05-408-129 )
- Cryogenic vials, 1.2 ml (Corning, catalog number: 430487 )
- TipOne RPT 10 µl ultra-low retention filter pipet tips, sterile (USA Scientific, catalog number: 1181-3810 )
- ARTTM Barrier pipette 100 µl tips 100E low retention (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2065E-05 )
- Human adipose tissue obtained from liposuction
- 1x phosphate-buffered saline (PBS) (GE Healthcare, HyCloneTM, catalog number: SH30256 )
- Trypan blue solution, 0.4% (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- Ethidium bromide
- Acridine orange
- 0.25% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200072 )
- Isopropanol 95% (v/v)
- Liquid nitrogen
- Type I collagenase (Worthington Biochemical, catalog number: LS004196 )
- Bovine serum albumin (BSA), fraction V (Sigma-Aldrich, catalog number: 10735078001 )
- Calcium chloride (CaCl2), ≥ 96.0% anhydrous (Sigma-Aldrich, catalog number: C4901 )
- Dulbecco’s modified Eagle medium (DMEM): Nutrient Mixture F-12 (DMEM/F-12) (Thermo Fisher Scientific, GibcoTM, catalog number: 11330032 )
- α-minimal essential medium (α-MEM), no nucleosides (Thermo Fisher Scientific, GibcoTM, catalog number: 12561056 )
- Fetal bovine serum (FBS), premium select (Atlanta Biologicals, catalog number: S11550 )
- Penicillin-streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- L-glutamine (200 mM) (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081 )
- DMSO
- Digestion solution (see Recipes)
- Resuspension solution (see Recipes)
- Complete culture medium (CCM) (see Recipes)
- Freezing medium (see Recipes)
Equipment
- Precision scale
- IsotempTM digital-control water bath (Fisher Scientific, model: 215 )
- Sterile cell culture hood
- Motorized pipette aid
- Micropipettes: 10 µl, 200 µl, and 1,000 µl (Eppendorf, model: Research® plus )
- Centrifuge (Eppendorf, model: 5810 R )
- Cell culture incubator
- Inverted routine light microscope (Nikon Instruments, model: Eclipse TS100 )
- Hemocytometer
- Nalgene® freezing container (Sigma-Aldrich, catalog number: C1562 )
- Cryogenic storage dewar (Custom BioGenic Systems, model: 6001 Value Added Cryosystem Dewar )
Procedure
- ASC isolation (Figure 1)

Figure 1. Schematic representation of the ASC isolation process. The lipoaspirate is separated into 50 ml conical tubes, blood is aspirated, washed with PBS, incubated with digestion solution, the digestion solution is neutralized, and the tube is centrifuged. Once the tube is centrifuged, the supernatant is discarded and the SVF is washed until it is white. The SVF is then resuspended, cells are counted, then plated on cell culture plates.- Warm water bath to 37 °C.
- Collect lipoaspirate from operating room in a sterile specimen collection container. Lipoaspiration samples commonly drain directly into vacuum containers in the operative field. These containers are sterile and can be safely transported to the laboratory where cell isolation can occur. Keep lipoaspirate at room temperature until ready to isolate cells and maintain sterile conditions when transferring to the tissue culture hood.
- Prepare digestion solution (see Recipe 1). Filter the solution under sterile conditions with a vacuum filter.
- Prepare resuspension solution (see Recipe 2). Filter the solution under sterile conditions with vacuum filter. Place resuspension solution in a 37 °C water bath.
- Warm digestion solution and PBS in the water bath to 37 °C.
- Place lipoaspirate and prepared solutions into a sterile cell culture hood and perform the following steps under sterile conditions.
- Separate lipoaspirate into sterile 50 ml centrifuge tubes by placing about 15 ml of lipoaspirate in each tube. Allow lipoaspirate to separate into blood and fat layers. The fat layer will float to the top of the tube, and the blood layer will be underneath it (see Figure 1).
- Pierce the fat layer with the aspiration pipette, and aspirate the blood layer using a 2 ml aspiration pipet. Avoid aspirating the fat layer, as this will clog the aspiration pipette (see Figure 1).
- Wash the fat layer with PBS until the lipoaspirate is a light reddish-yellow hue. The volume of PBS required will vary depending on how much blood is present in the lipoaspirate sample. The volume of PBS needed will range from 5 to 20 ml, and multiple washes will be required until the wash solution is clear. Centrifuge the solution at 300 x g at room temperature for 5 min to separate the wash from the fat layer.
- Aspirate the PBS wash below the fat layer, and avoid aspirating the fat layer and the cell pellet at the bottom of the centrifuge tube (see Figure 1).
- Incubate lipoaspirate in a volume of digestion solution equivalent to the original amount of lipoaspirate (15 ml) for 60 min at 37 °C. For example, 15 ml of lipoaspirate should be incubated in 15 ml of digestion solution. Mix the tube(s) by vigorously shaking the tube for 1 min and mix the tubes intermittently every 5 to10 min throughout the digestion time.
- Neutralize collagenase by adding an equal amount of resuspension solution to the digestion solution used. For example, 15 ml of lipoaspirate incubated in 15 ml of digestion solution should be neutralized by 15 ml of resuspension solution.
- Centrifuge the solution at 300 x g at room temperature for 5 min. This step separates floating adipocytes, oil, fat, collagenase solution and the SVF.
- Discard supernatant, leaving about 5 ml of solution and the SVF cell pellet. Resuspend the SVF cell pellet in 10 ml PBS.
- Repeat steps A12 and A13 two times or until pellet appears white. These washes remove red blood cells and other non-adherent cells without compromising the yield of ASCs.
- Resuspend the SVF in 5 ml of resuspension solution. Pipet 10 µl of cells into a 1.5 ml microcentrifuge tube.
- Add 10 µl of trypan blue. Mix the solution with the cells by pipetting up and down 3-5 times.
- Add 10 µl of the mixed trypan blue-cell solution to the hemocytometer. Count cells in the four outer quadrants.
- As an alternative to trypan blue, mix 1 µl of 1:1 solution of 100 µg/ml ethidium bromide and 100 µg/ml acridine orange with 25 µl of cells. Add 10 µl of the mixture to the hemocytometer. Count the cells under fluorescent microscopy.
- Use the following equations to determine the total number of cells per milliliter and the total number of cells:
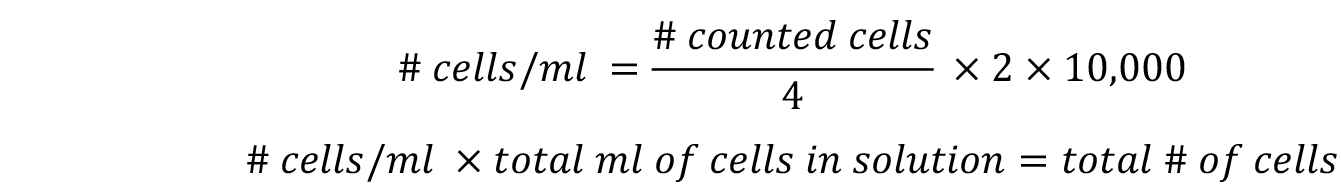
Where,
‘2’ represents the dilution factor,
‘10,000’ represents the hemocytometer constant. - Plate the cells on 145 cm2 culture dishes at a density of 100 cells per cm2 in 20 ml of CCM (approximately 14,500 cells per 145 cm2 culture dish). The expected cell yield ranges from 1.6 x 105 to 1.1 x 106 cells per ml of liposuction tissue.
- Maintain cells in a humidified 5% CO2 incubator at 37 °C for 24-72 h. Since donor variability is expected with human samples, the variability in initial incubation duration is associated with differences in the rate at which different donors’ cells will adhere to the plate.
- After 24-72 h, gently wash cell layer with PBS to remove non-adherent cells, leaving behind the adherent ASCs on the plate.
- Aspirate and discard PBS wash.
- Replace medium with CCM (15 to 20 ml for 145 cm2 plate) following the aspiration of PBS. Replace the medium with CCM every 2 to 3 days until cells have reached 70-80% confluence. Time to confluency will depend on the proliferative rate of the cells. Confluency of 80% can be expected within 4 to 10 days with medium replacements every 2-3 days. The cells should be passaged (see Procedure B) or cryopreserved (see Procedure C) once sufficient proliferation has occurred.
- Visualize cells under light microscopy to check for the absence of floating cells. The presence of floating cells indicates poor adherence and cell death.
- Continue to maintain cells in a humidified 5% CO2 incubator at 37 °C for 24-72 h.
- Warm water bath to 37 °C.
- Expansion of ASCs
- Visualize the cells under light microscopy (see Figure 2). The cells should be approximately 80% confluent at the time of expansion. This confluence level is optimal as it prevents changes in cytokine expression that occurs when ASCs overgrow.
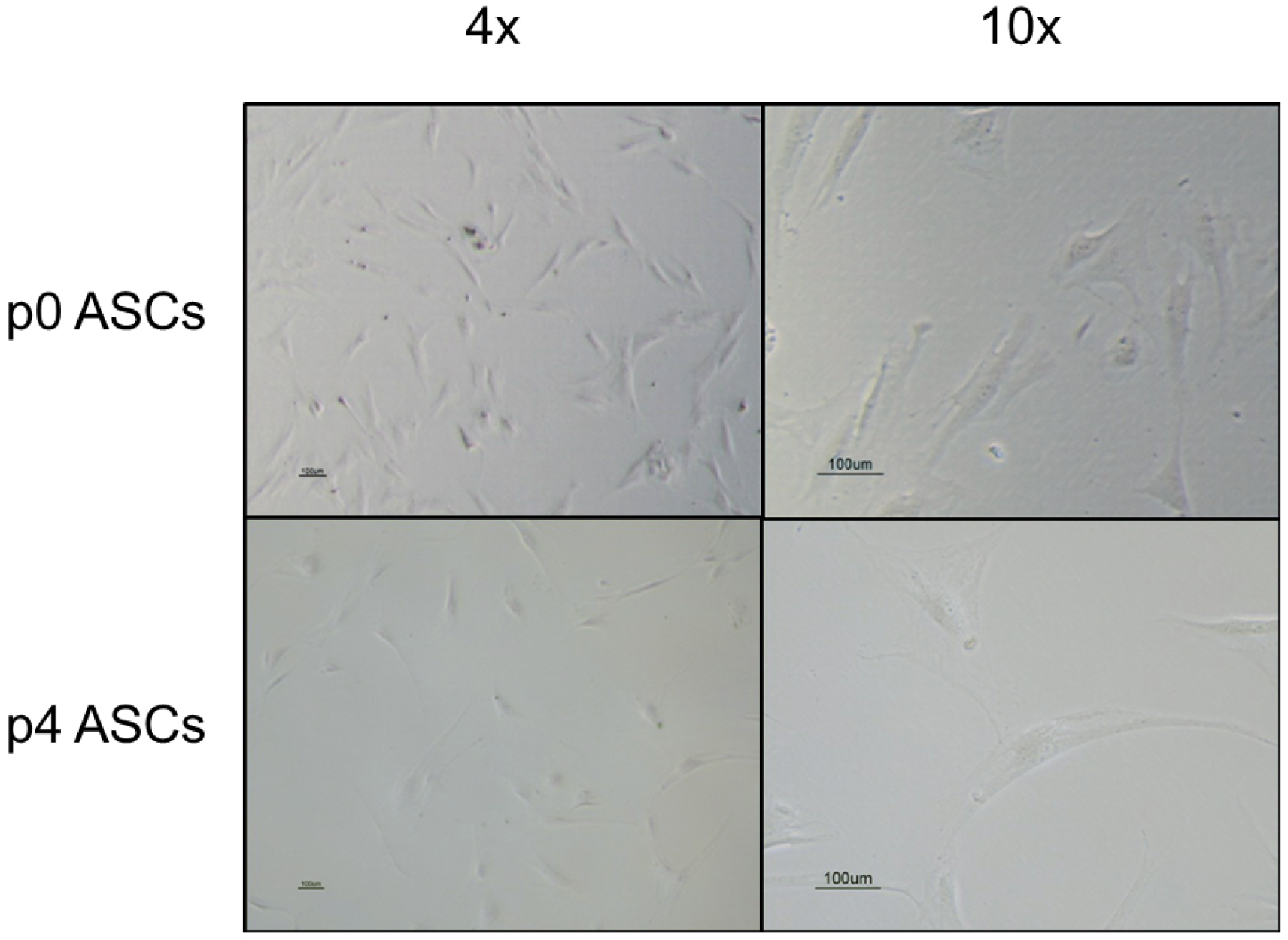
Figure 2. Light microscopy of passage (p) 0 and p4 ASCs under 4x and 10x magnification. The ASCs are identified by their spindle-shaped morphology. Scale bars = 100 μm. - Warm 0.25% trypsin-EDTA, media, and PBS in 37 °C water bath.
- Aspirate off CCM from the cell culture plate.
- Wash cells with 10 ml PBS and aspirate the wash.
- Add 2-5 ml of 0.25% trypsin to a 145 cm2 plate to adequately cover the entire area.
- Place 145 cm2 plates in the incubator set to 37 °C for a minimum of 5 min and no more than 20 min as cells will begin to lift into solution.
- Visualize the cells under light microscopy to determine whether the enzymatic reaction is complete and the cells have lifted. Neutralize the trypsin by adding an equal volume of CCM. The reaction should not take longer than 10 min and should be neutralized if 10 min have passed to prevent degradation of the cell membrane.
- Pipet the cell solution into a sterile 50 ml conical centrifuge tube and spin at 300 x g for 5 min at room temperature.
- Aspirate the supernatant, leaving behind the cell pellet.
- Resuspend the cell pellet in CCM.
- Plate cells at a density of 100 cells per cm2.
- Visualize the cells under light microscopy (see Figure 2). The cells should be approximately 80% confluent at the time of expansion. This confluence level is optimal as it prevents changes in cytokine expression that occurs when ASCs overgrow.
- Cryopreservation of ASCs
- Remove the vial compartment of the freezing container and ensure that the freezing container is filled with isopropanol to the appropriate volume.
- Prepare freezing medium (see Recipe 4).
- Aspirate the media, wash with 10 ml PBS, and aspirate the PBS. The PBS washes away residual sera which inhibits trypsin.
- Add 2-5 ml of 0.25% trypsin to a 145 cm2 plate to adequately cover the entire area.
- Place cells in a 37 °C cell culture incubator for 5 min.
- Visualize the cells under light microscopy to determine whether the enzymatic reaction is complete and the cells have lifted. Neutralize the trypsin by adding an equal volume of CCM. The reaction should not take longer than 10 min and should be neutralized if 10 min has passed.
- Centrifuge 300 x g for 5 min at room temperature.
- Resuspend in 1 ml PBS. Count cells using the hemocytometer method as above.
- Centrifuge 300 x g for 5 min at room temperature.
- Aspirate supernatant and resuspend cells in freezing media to approximately 1 x 106 cells/ml.
- Pipet 1 ml of cell suspension into clean 1.2 ml cryogenic vials. Using this volume of cell suspension, each cryogenic vial will have approximately 1 x 106 frozen cells. Label vials with appropriate sample titles and date of freezing. Once the cells are in the freezing media, move vials to -80 °C freezer within 5 min. Extended exposure to DMSO at room temperature can lead to decreased cell viability.
- Place vials into an alcohol jacketed freezing container. The alcohol jacketed container slows the rate of cooling to about -1 °C/min, which is the ideal rate of cooling for cell preservation.
- Place alcohol jacketed freezing container into -80 °C freezer.
- Once the cells have been in the freezer for 24 h, they may be moved into liquid nitrogen.
- Remove the vial compartment of the freezing container and ensure that the freezing container is filled with isopropanol to the appropriate volume.
Data analysis
For representative photos and results, please refer to Estes et al. (2010).
Notes
- Using cells from lower passages leads to better reproducibility. Therefore, we recommend using ASC between passages 2-6 for all experiments. Passage 1 cells represent the initial population of ASCs frozen down after isolation.
- Prepare all solutions the day of or the day prior to use for best results.
- Cell isolation should be performed the same day as tissue harvest from the patient for the highest viability, though our group has also determined that > 90% of adherent ASCs can be recovered from tissue as long as 24 h after collection.
- All lipoaspirate samples must be obtained according to Institution Review Board policies regarding human tissue collection. These protocols include informed consent from patients and tissue manipulation only by those who have been trained to work with blood borne pathogens.
Recipes
- Digestion solution
100 ml PBS
0.1 g collagenase I
1 g BSA stock
200 µl of 200 mM CaCl2
Gently rock tube until solutions are homogenous and CaCl2 has dissolved completely
Sterile filter immediately prior to use - Resuspension solution
900 ml DMEM/F-12
100 ml FBS - Complete culture medium (CCM) (1,000 ml)
780 ml α-MEM
200 ml FBS
10 ml 100x L-glutamine
10 ml penicillin-streptomycin 10,000 U/ml - Freezing medium (100 ml)
65 ml of α-MEM
30 ml of FBS
5 ml of DMSO
Filter in sterile filter unit
Note: DMSO should be added to the medium and allowed to cool before adding the sera. Be sure to use a DMSO safe filter unit. Once thawed, discard any unused medium.
Acknowledgments
This protocol is an expansion of the protocol listed in Strong et al. (2016). The authors would like to thank Marjorie McCants for assisting with organization of materials and equipment, and Stephen Dickinson, Annie Bowles, and Rachel Sabol for helping with creation of the figures. J.M.G. is co-founder and co-owner of LaCell, LLC. All other authors indicate no conflicts of interest.
References
- Aust, L., Devlin, B., Foster, S. J., Halvorsen, Y. D., Hicok, K., du Laney, T., Sen, A., Willingmyre, G. D. and Gimble, J. M. (2004). Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6(1): 7-14.
- Estes, B. T., Diekman, B. O., Gimble, J. M. and Guilak, F. (2010). Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc 5(7): 1294-1311.
- Gimble, J. and Guilak, F. (2003). Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5(5): 362-369.
- Strong, A. L., Bowles, A. C., Wise, R. M., Morand, J. P., Dutreil, M. F., Gimble, J. M. and Bunnell, B. A. (2016). Human adipose stromal/stem cells from obese donors show reduced efficacy in halting disease progression in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Stem Cells 34(3): 614-626.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jones, R. B., Strong, A. L., Gimble, J. M. and Bunnell, B. A. (2017). Isolation and Primary Culture of Adult Human Adipose-derived Stromal/Stem Cells. Bio-protocol 7(5): e2161. DOI: 10.21769/BioProtoc.2161.
Category
Stem Cell > Adult stem cell > Adipose Stem Cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












