- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
VLA-4 Affinity Assay for Murine Bone Marrow-derived Hematopoietic Stem Cells
Published: Vol 7, Iss 4, Feb 20, 2017 DOI: 10.21769/BioProtoc.2134 Views: 8874
Reviewed by: Varpu MarjomakiRalph BottcherVivien Jane Coulson-Thomas

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Ex Vivo Testing of CD8+ T-Cell Division and Activation Using Mouse Splenocytes
Melissa Dolan [...] John M.L. Ebos
Aug 20, 2025 3878 Views

Detection of Autophagy in Human Peripheral Blood Mononuclear Cells Using Guava® Autophagy and Flow Cytometry
Melanie Scherer [...] Jörg Bergemann
Sep 20, 2025 1417 Views

Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
Nov 5, 2025 1448 Views
Abstract
Hematopoietic stem cells (HSCs) are defined by their functional ability to self-renew and to differentiate into all blood cell lineages. The majority of HSC reside in specific anatomical locations in the bone marrow (BM) microenvironment, in a quiescent non motile mode. Adhesion interactions between HSCs and their supporting BM microenvironment cells are critical for maintaining stem cell quiescence and protection from DNA damaging agents to prevent hematology failure and death. Multiple signaling proteins play a role in controlling retention and migration of bone marrow HSCs. Adhesion molecules are involved in both processes regulating hematopoiesis and stem- and progenitor-cell BM retention, migration and development. The mechanisms underlying the movement of stem cells from and to the marrow have not been completely elucidated and are still an object of intense study. One important aspect is the modification of expression and affinity of adhesion molecules by stem and progenitor cells which are required both for stem cell retention, migration and development. Adhesion is regulated by expression of the adhesion molecules, their affinity and avidity. Affinity regulation is related to the molecular binding recognition and bond strength. Here, we describe the in vitro FACS assay used in our research to explore the expression, affinity and function of the integrin α4β1 (also termed VLA-4) for murine bone marrow retained EPCR+ long term repopulation HSC (LT-HSC) (Gur-Cohen et al., 2015).
Keywords: Hematopoietic stem cellsBackground
Integrins are type I transmambrane glycoprotein receptors that mediate cell-cell and cell-matrix adhesion interactions, signaling and communication. All integrins are heterodimers of non-covalently associated α and β subunits. In humans integrin heterodimers are formed from 9 types of β subunits and 24 types of α subunits. This diversity is further increased by alternative splicing of some integrin RNAs. Each heterodimer consists of a large extracellular domain which binds proteins in the extracellular environment, a single transmembrane domain, and an intracellular cytoplasmic tail domain. The largest integrin subfamily is composed by integrin β1 (CD29) that is able to associates with 12 different α subunits (α1-11 and αv). Integrin β1 together with integrin chains α4, α5, α6 and α9 are expressed by murine hematopoietic stem and progenitor cells (HSPCs) and play important roles in regulating their BM retention, migration and development.
Integrins, like other transmembrane receptors, display an ‘outside-in signaling’, i.e., to transduce the signal intracellularly after the binding with their ligand. Moreover, integrins have a peculiar feature: they are able to shift between high- and low-affinity conformation states for ligand binding (‘inside-out’ signaling) (Takagi and Springer, 2002). According to the cell type, integrins can be either basically activated or basally inactive. In the inactive state, the integrin extracellular domains are not bounded to the ligands, and are in a bent conformation. Following intracellular activation signals, the extracellular domain is straightened, stabilizing the extended active conformation. Thus, the external ligand binding site, is now exposed to the ligand binding, allowing the transmission of the signals from the outside to the inside (Luo et al., 2007).
Very Late Antigen-4 (VLA-4, also known as CD49d/CD29 or α4β1) is a member of the integrin α4 family together with α4β7. Within the integrin family, VLA-4 has some unique features. In contrast to related members of β1 subfamily, VLA-4 is predominantly expressed on hematopoietic lineage cells (Hemler, 1990) and is functionally involved in both cell-cell and cell-ECM adhesive interactions. Moreover, despite sequence homology with other integrin α subunits, the α4 strand because of the lack of the inserted I-domain doesn’t undergo post-translational cleavage near the transmembrane region. Finally, the α4 chain contains a trypsin-like cleavage site, constitutively expressed on most leukocytes and on hematopoietic stem and progenitor cells (Hynes, 1992).
VLA-4 plays a major role in the regulation of immune cell recruitment to inflamed endothelia and sites of inflammation through its interactions with two alternative ligands, vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced connecting segment 1 (CS-1) of fibronectin (Hemler, 1990; Papayannopoulou et al., 1998). It also participates in many cellular events and is crucial for BM retention and mobilization of immature stem and progenitors cells from the bone marrow (Lapidot and Petit, 2002; Peled et al., 2000).
Migration of hematopoietic stem cells to the bone marrow is a regulated multistep process that requires precise regulation and activation of various molecules including chemoattractants, selectins and integrins. While the initial steps of hematopoietic stem and progenitor cells tethering and rolling along BM blood vessel endothelium are primarily regulated by selectins, various integrins have been shown to be involved in the next stages of this process. VLA-4 plays an important role in homing, lodgment and retention of HSCs within the marrow microenvironment (Rettig et al., 2012). Previous studies demonstrate that treatment of donor BM cells with a neutralizing anti α4 integrin antibody before injection into lethally irradiated recipients, inhibits their homing to the femurs of recipient mice, increasing the number of HSPCs in the peripheral blood and spleen. Moreover, recipient mice pretreated with neutralizing antibodies against VCAM-1 gave similar results, adding evidences to the important role of the VLA-4/VCAM-1 axis in HSPC homing to the bone marrow (Papayannopoulou et al., 1995).
Recently, factors traditionally related to coagulation and inflammation have been shown to independently control long term (LT) HSCs retention in the bone marrow and their recruitment to the blood (Aronovich et al., 2013; Gur-Cohen et al., 2015). Adult murine BM LT-HSCs, endowed with the highest repopulation and self-renewal potential, express endothelial protein C receptor (EPCR) which is used as a marker to isolate them (Balazs et al., 2006).
Protease-activated receptor-1 (PAR1) is functionally expressed by bone marrow stromal and endothelial cells as well as HSC and immature and maturing leukocytes (Gur-Cohen et al., 2016). Activated protein C ([aPC], the major ligand for EPCR)-EPCR/PAR1 signaling facilitate LT-HSC BM repopulation, retention, survival, and chemotherapy resistance by restricting nitric oxide (NO) production. Inhibition of NO generation by aPC/EPCR/PAR1 signaling on LT-HSC, inhibits downstream CDC42 activity and induces CDC42 polarity, as well as increasing VLA4 expression, affinity and adhesion. Conversely, acute stress and clinical mobilization up-regulate thrombin generation and activate different PAR1 signaling leading to NO generation that overcomes BM EPCR+LT-HSC retention, inducing TACE mediated EPCR and VLA-4 shedding, up-regulation of CXCR4 and PAR1 on LT-HSC, stromal PAR1 mediated CXCL12 secretion, resulting in stem and progenitor cell recruitment to the blood stream (Gur-Cohen et al., 2015). VLA-4 is expressed at a higher level by bone marrow EPCR+LT-HSC together with higher affinity to its ligands, inducing their BM retention and protection from DNA damaging agents. The restriction of NO by EPCR/PAR1 signaling increase the affinity of VLA-4 regulating anchorage and bone marrow retention of LT-HSC and chemotherapy resistant (Gur-Cohen et al., 2015).
Multiple small molecules have been developed in an attempt to regulate integrin dependent adhesion. The affinity states of human VLA-4 can be recognized by monoclonal antibodies sensitive to its molecular conformation (Masumoto and Hemler, 1993). Importantly, changes in VLA-4 affinity can be detected in real-time and on a physiologically relevant time frame using a ligand mimicking LDV-containing fluorescent small molecule (LDV-FITC) by FACS (Chigaev et al., 2001). VLA-4 recognize with high affinity a peptide sequence within fibronectin, which comprises 25 amino acid, termed CS-1 (Hynes, 1992). LDV (leu-asp-val) is the tripeptide identified as the minimal sequence for specific VLA-4 recognition of CS-1 segment of fibronectin. Here we describe a method to detect VLA-4 affinity monitoring mean fluorescent intensity through flow cytometry using LDV-FITC.
Materials and Reagents
- Syringe with needle 1 ml 25 G x 5/8 in. (0.5 x 16 mm) (BD, catalog number: 300014 )
- Dishes 35 x 10 (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 153066 )
- 70 μm nylon strainer (Sinun Tech, catalog number: Polymer Screens )
- FACS tubes (Corning, FalconTM, catalog number: 352054 )
- Cells of interest (here murine bone marrow cells obtained from 8 weeks old mice)
- Dulbecco’s phosphate-buffered saline (PBS+/+) (Biological Industries, catalog number: 02-020-1A )
- EDTA (5 mM final concentration in water, pH 7.4) (Avantor® Performance Materials, J.T.Baker, catalog number: 8993-01 )
- Wet ice
- Antibodies to detect LT-HSCs by flow cytometry:
- Sca-1 PEcy7 (clone D7) (Biolegend, catalog number: 108114 )
- c-kit APC (clone 2B8) (Biolegend, catalog number: 105812 )
- CD150 Brilliant Violet (clone TC15-12F12.2) (Biolegend, catalog number: 115922 )
- CD48 Pasific Blue (clone HM48-1) (Biolegend, catalog number: 103418 )
- EPCR PE (clone eBio1560) (Affymetrix, eBioscience, catalog number: 12-2012-82 )
- Lineage: CD4 (clone GK 1.5), CD8a (clone 53-6.7), GR1 (clone RB6-8C5), B220 (clone RA3-682), Ter119 (clone TER-119), CD11b (clone M1/70)
- Calcium chloride dihydrate (EMD Millipore, catalog number: 102382 )
- Magnesium chloride hexahydrate (EMD Millipore, catalog number: 105833 )
- HEPES buffer solution (Biological Industries, catalog number: 03-025-1B )
- Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14175095 )
- Bovine serum albumin solution (10%, BSA) (Biological Industries, catalog number: 03-010-1B )
- 2.4-((N’-2-methylphenyl)ureido)-phenylacetyl-L-leucyl-L-aspartyl-L-valyl-L-prolyl-L-alanyl-L-alanyl-L-lysine (LDV-FITC) (R&D System, catalog number: 4577 )
- Deionized water (DDW)
- Gentian violet (Sigma-Aldrich, catalog number: 548-62-9 )
- Acetic acid (Sigma-Aldrich, catalog number: 64-19-7 )
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 30525-89-4 )
- LDV medium (see Recipes)
- LDV-FITC stock and working solutions (see Recipes)
- Turk’s solution (see Recipes)
- 4% PFA (see Recipes)
Note: Flurochrome should be chosen according to the flow cytometry machine.
Equipment
- Forceps (Kent Scientific, catalog number: INS700100-2 )
- Scissors (Kent Scientific, catalog number: INS750046 )
- Centrifuge (Eppendorf, model: 5810R )
- Centrifuge swing-bucket rotor A-462 4 x 250 ml rectangular buckets (Eppendorf, catalog number: 5810709008 )
- Adapters (Eppendorf, catalog number: 5810752000 )
- Inverted light microscope (Olympus, model: CHK2-F-GS )
Note: This product has been discontinued by the manufacturer. - Hemocytometer (Sigma-Aldrich, Bright-LineTM, catalog number: Z359629 )
- Incubator 37 °C, 5% CO2 (Thermo Fisher Scientific, Thermo ScientificTM, model: 150i )
- Cold room or refrigerator (4 °C)
- Flow cytometer (i.e., Macs Quant instrument [Miltenyi, BergischGladbach, Germany] or BD LSR II flow cytometer)
- 2 L glass flask or bottle (Kimble Chase Life Science and Research Products, catalog number: 26500-2000 )
- Plastic weigh boat (Sigma-Aldrich, catalog number: Z186856 )
Note: Color combinations can be adjusted to match the laser combinations available.
Software
- FlowJo V10 software (Tree Star, optional)
Procedure
- Obtain murine bone marrow cells
Note: All animal experiments have to be approved by local animal care and ethics authorities.- Sacrifice desired mice strain by CO2 euthanization or cervical dislocation.
- Extract the femurs, tibias and iliac crest bones using forceps and sharp scissors. Place the bones in a small dish supplemented with PBS+/+ on ice.
- Flush total bone marrow cells from the bone cavity with 1-5 ml ice cold PBS (per mouse) using a 1 ml syringe and 25 G needle. Obtain single cell suspension by simply resuspending the cell solution using the same syringe.
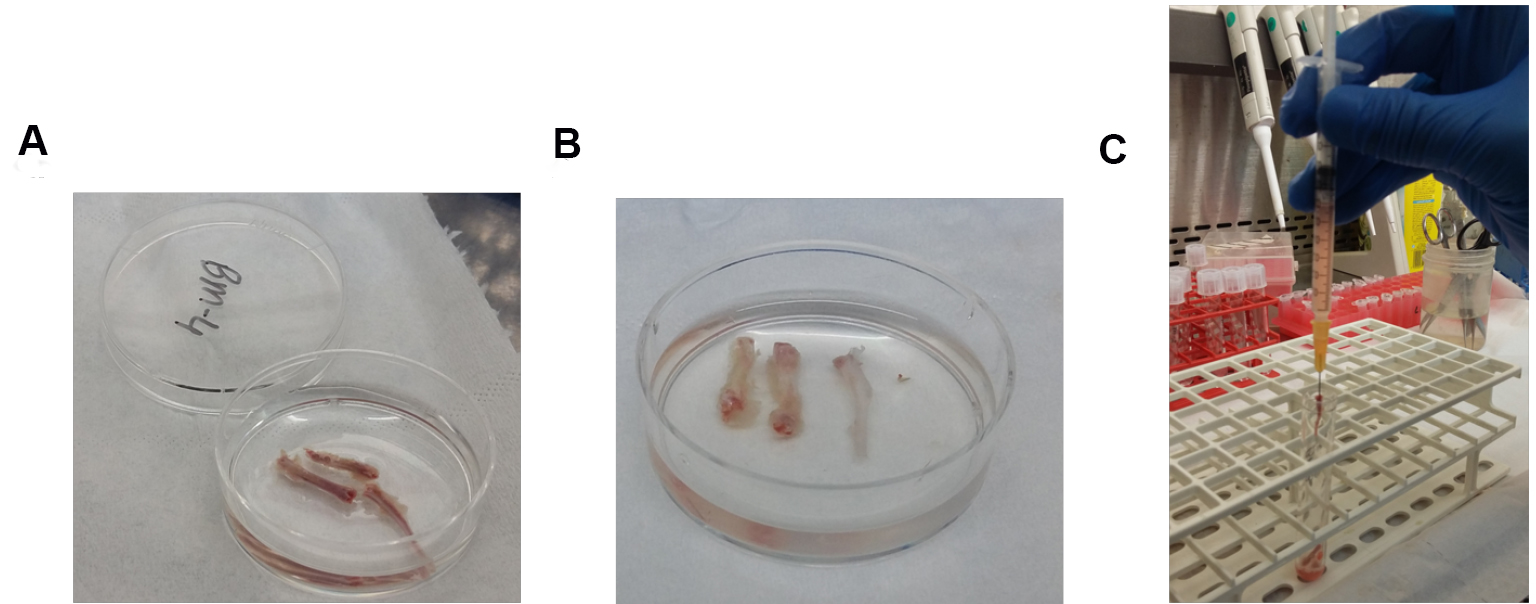
Figure 1. Process of flushing bone marrow out of murine bones. A. Femurs, tibias and iliac crest bones before flushing; B. Femurs, tibias and iliac crest bones after flushing; C. 1 ml syringe with its 25 G needle inserted in the tibia bone cavity. - Filter the cells by passing the cell solution through 70 μm nylon strainer to obtain a uniform single-cell suspension.
- Centrifuge the cells (289.5 x g, 5 min) and resuspend in 1 ml LDV medium.
- Count the cells by diluting the cells 1:10 with Turk’s solution. Count the cells under the inverted light microscope using the hemacytometer. From one mouse typically one will get 60-90 million cells from both femurs and tibias and iliac crest bones.
- Sacrifice desired mice strain by CO2 euthanization or cervical dislocation.
- Determination of VLA-4 affinity using flow cytometry (LDV assay)
VLA-4 affinity can be examined on fresh bone marrow cells obtained from treated animals. Alternatively, cells can be obtained from untreated animals and treated in vitro with a desired reagent, in the presence of LDV medium, followed by the abovementioned detailed protocol.
Note: It is recommended to continue with the staining after isolation of the bone marrow cells.- Place 5 million bone marrow cells in a total volume 100 μl of LDV medium (see Recipes) in a FACS tube.
- Add LDV-FITC to achieve a final concentration of 10 nM (see Recipes) and mix gently by pipetting, do not vortex.
- To determine LDV-FITC non-specific binding, add EDTA to each LDV-FITC sample at a final concentration of 5 mM.
- Incubate all the samples for 30 min at 37 °C.
- After incubation, put the tubes immediately on wet ice and fix the cells by adding ice cold 4% PFA for 10 min (i.e., the reaction is in 100 µl of cells, thus add 4% PFA [1-2 ml] directly to the tube). Since that moment, cells need to be kept under cold conditions on wet ice. Avoid vortex along the procedure.
- Wash the cells with 2 ml ice cold PBS+/+.
- Centrifuge the cells (289.5 x g, 5 min) and gently discard the supernatant.
- Add 100 µl of ice cold PBS+/+ and perform staining of the cell surface markers Lineage (1-2 µl per sample of each antigen), Sca-1 (2 µl/sample), c-Kit (2 µl/sample), CD150 (2 µl/sample), CD48 (1 µl/sample) and EPCR (2 µl/sample). These cell surface markers are only suggestive antigens to detect LT-HSCs, however, this can be adjusted according to the research question needs. Color combination should fit with FITC as LDV peptide is conjugated with the FITC fluorochrome.
- Add the required antibodies into the cell samples and mix gently by pipetting (do not vortex), and incubate for 30 min on ice and protected from light.
- Wash the cells with 1 ml ice cold PBS+/+, centrifuge (289.5 x g, 5 min) and gently discard the supernatant.
- Resuspend the cells in 300 μl PBS+/+, do not vortex.
- Read the samples immediately by flow cytometry and determine LDV binding to LT-HSC by analyzing the intensity of the FITC fluorochrome on gated Lineage-/c-Kit+/Sca-1+/CD150+/CD48-/EPCR+ cells.
- Place 5 million bone marrow cells in a total volume 100 μl of LDV medium (see Recipes) in a FACS tube.
Data analysis
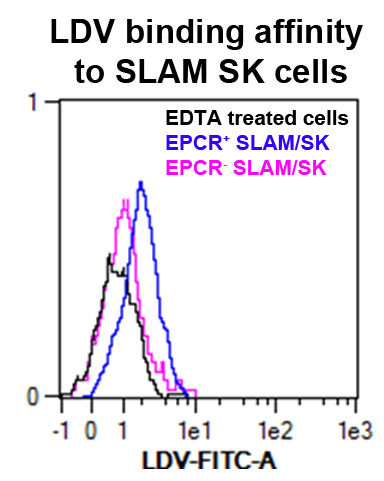
Figure 2. VLA4 affinity measured by LDV probe binding to bone marrow EPCR+ SK (Sca1+ ckit+) SLAM (CD150+ CD48-) cells. In black is the control cells treated with EDTA; in blue the EPCR+ cells gated on SLAM/SK; in pink the EPCR-cells gated on SLAM/SK.
For further analysis information concerning gating strategies and statistical analysis you can consult the article Gur-Cohen et al., 2015 at the following link: http://www.nature.com/nm/journal/v21/n11/full/nm.3960.html.
Recipes
- LDV medium
1 mM CaCl2
1 mM MgCl2
20 mM HEPES, containing 1% BSA
Note: It is recommended to prepare stock solutions of 1 M CaCl2 (dilute 1:1,000 directly in the medium) as well as 100 mM MgCl2 (dilute 1:100 directly in the medium). - LDV-FITC stock and working solutions
Dissolve 1 mg LDV-FITC powder in 1 ml DDW (ddH2O) according to the manufacturer's instructions (giving a stock concentration of 0.73 mM)
Note: It is recommended to divide the solution into small aliquots, avoiding repeated freeze/thaw cycles (the solution can be stored at -20 °C for up to 1 year).
To prepare the working LDV-FITC solution, dilute stock solution with DDW, reaching to a final concentration of 10 nM (for 10 nM working solution dilute 1:730 with DDW and take 1 µl into 100 µl cells supplemented with LDV medium) - Turk solution
50 mg gentian violet
5 ml acetic acid
495 ml DDW
Dissolve gentian violet in acetic acid and DDW - 4% PFA
Weight out 40 g of powdered paraformaldehyde into a plastic weigh boat
Pour into a 2 L glass flask or bottle
Add 1 L DDW, a stir bar and allow to gently agitate while warming to 65 °C, for 5 min
Add one drop of 10 N KOH or 10 N NaOH base, the solution should then become clear
Allow the solution to cool to room temperature and adjust the pH to 7.3
Note: Smaller volumes can be stored at -20 °C for up to one year.
Acknowledgments
This study was supported by the Israel Science Foundation (851/13), the Ernest and Bonnie Beutler Research Program of Excellence in Genomic Medicine and FP7-HEALTH-2010 (CELL-PID 261387) (T.L.) and the DKFZ, Germany.
References
- Aronovich, A., Nur, Y., Shezen, E., Rosen, C., ZlotnikovKlionsky, Y., Milman, I., Yarimi, L., Hagin, D., Rechavi, G., Martinowitz, U., Nagasawa, T., Frenette, P. S., Tchorsh-Yutsis, D. and Reisner, Y. (2013). A novel role for factor VIII and thrombin/PAR1 in regulating hematopoiesis and its interplay with the bone structure. Blood 122(15): 2562-2571.
- Balazs, A. B., Fabian, A. J., Esmon, C. T. and Mulligan, R. C. (2006). Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood 107(6): 2317-2321.
- Chigaev, A., Blenc, A. M., Braaten, J. V., Kumaraswamy, N., Kepley, C. L., Andrews, R. P., Oliver, M. J., Edwards, S. B., Prossnitz, S. R., Larson, S. R. and Sklar, L. A. (2001). Real time analysis of the affinity regulation of α4-Integrin: the physiologically activated receptor is intermediate in affinity between resting and Mn2+ or antibody activation. JBC 276(52): 48670-48678.
- Gur-Cohen, S., Itkin, T., Chakrabarty, S., Graf, C., Kollet, O., Ludin, A., Golan, K., Kalinkovich, A., Ledergor, G., Wong, E., Niemeyer, E., Porat, Z., Erez, A., Sagi, I., Esmon, C. T., Ruf, W. and Lapidot, T. (2015). PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat Med 21(11): 1307-1317.
- Gur-Cohen, S., Kollet, O., Graf, C., Esmon, C. T., Ruf, W. and Lapidot, T. (2016). Regulation of long-term repopulating hematopoietic stem cells by EPCR/PAR1 signaling. Ann N Y AcadSci 1370(1): 65-81.
- Hemler, M. E. (1990). VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Ann Rev Immunol 8(1): 365-400.
- Hynes, R. O. (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69(1): 11-25.
- Lapidot, T. and Petit, I. (2002). Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 30(9): 973-981.
- Luo, B. H., Carman, C. V. and Springer, T. A. (2007). Structural basis of integrin regulation and signaling. Annu Rev Immunol 25: 619-647.
- Masumoto, A. and Hemler, M. E. (1993). Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem 268(1): 228-234.
- Papayannopoulou, T., Craddock, C., Nakamoto, B., Priestley, G. V. and Wolf, N. S. (1995). The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A 92(21): 9647-9651.
- Papayannopoulou, T., Priestley, G. V. and Nakamoto, B. (1998). Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood 91(7): 2231-2239.
- Peled, A., Kollet, O., Ponomaryov, T., Petit, I., Franitza, S., Grabovsky, V., Slav, M. M., Nagler, A., Lider, O., Alon, R., Zipori, D. and Lapidot, T. (2000). The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34+ cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95(11): 3289-3296.
- Rettig, M. P., Ansstas, G. and Di Persio, J. F. (2012). Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia 26(1): 34-53.
- Takagi, J. and Springer, T. A. (2002). Integrin activation and structural rearrangement. Immunol Rev 186: 141-163.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Avemaria, F., Gur-Cohen, S., Avci, S. and Lapidot, T. (2017). VLA-4 Affinity Assay for Murine Bone Marrow-derived Hematopoietic Stem Cells. Bio-protocol 7(4): e2134. DOI: 10.21769/BioProtoc.2134.
Category
Stem Cell > Adult stem cell > Hematopoietic stem cell
Cell Biology > Cell-based analysis > Flow cytometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








