- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Enriching Acidophilic Fe(II)-oxidizing Bacteria in No-flow, Fed-batch Systems
Published: Vol 7, Iss 3, Feb 5, 2017 DOI: 10.21769/BioProtoc.2130 Views: 9348
Reviewed by: Darrell CockburnAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Glucose Starvation, Magnesium Ion Starvation, and Bile Stress Assays
Aryashree Arunima and Mrutyunjay Suar
Sep 20, 2021 2971 Views

Analysis of Heterocyst and Akinete Specific Glycolipids in Cyanobacteria Using Thin-layer Chromatography
Ritu Garg [...] Iris Maldener
Mar 20, 2022 2461 Views

Detecting Photoactivatable Cre-mediated Gene Deletion Efficiency in Escherichia coli
Yuta Koganezawa [...] Miki Umetani
Jun 5, 2023 1946 Views
Abstract
Low-pH microbial Fe(II) oxidation occurs naturally in some Fe(II)-rich acid mine drainage (AMD) ecosystems across so-called terraced iron formations. Indigenous acidophilic Fe(II)-oxidizing bacterial communities can be incorporated into both passive and active treatments to remove Fe from the AMD solution. Here, we present a protocol of enriching acidophilic Fe(II)-oxidizing bacteria in no-flow, fed-batch systems. Mixed cultures of naturally occurring microbes are enriched from the fresh surface sediments at AMD sites using a chemo-static bioreactor (Eppendorf BioFlo®/Celligen® 115 Fermentor) with respect to constant stirring speed, temperature, pH and unlimited dissolved oxygen. Ferrous sulfate is discontinuously added to the reactor as the primary substrate to enrich for acidophilic Fe(II)-oxidizing bacteria. Successfully and efficiently enriching acidophilic Fe(II)-oxidizing bacteria helps to exploit this biogeochemical process into AMD treatment systems.
Keywords: Acid mine drainageBackground
Low-pH microbial Fe(II) oxidation can be incorporated into AMD passive treatment systems by enhancing the development of terraced iron formations (DeSa et al., 2010; Brown et al., 2011; Larson et al., 2014a and 2014b). For extremely difficult-to-treat AMD (very low pH, very high concentrations of Fe(II) and associated metals), an active treatment bioreactor is required by enriching acidophilic Fe(II)-oxidizing bacterial communities. This process can effectively change a high acidity, high metals discharge into a moderate acidity (still low pH), low metals discharge (Sheng et al., 2016).
Acidophilic aerobic Fe(II) oxidizers Acidithiobacillus spp., Leptospirillum spp., and Ferrovum myxofaciens have all been enriched in both suspended growth and fixed-film laboratory-scale bioreactors for AMD treatment (Hedrich and Johnson, 2012; Heinzel et al., 2009a and 2009b; Janneck et al., 2010; Tischler et al., 2013). For instance, Hedrich and Johnson (2012) designed an AMD remediation system that integrated low-pH Fe(II) oxidation and Fe removal in a multi-reactor system. A pure culture of the Fe(II)-oxidizer Ferrovum myxofaciens was enriched in first suspended-growth reactor. Heinzel et al. (2009a and 2009b), Janneck et al. (2010) and Tischler et al. (2013) all developed a natural mixed community of Fe(II)-oxidizers with porous fixed-film media in a pilot-scale reactor. A protocol of enriching mixed culture acidophilic Fe(II)-oxidizing bacteria in no-flow, fed-batch systems without fixed-film media is suggested here in a chemostatic bioreactor with controlled hydrogeochemical conditions (Sheng et al., 2016).
Materials and Reagents
- Sterile plastic containers
- 0.45 μm sterile bottle-top filters (Corning, catalog number: 430514 )
- Al foil
- 50 ml sterile centrifuge tubes (VWR, catalog number: 89039-656 )
- 15 ml sterile centrifuge tubes (VWR, catalog number: 89039-664 )
- 100% N2
- 0.1% (m/v) sodium pyrophosphate (EMD Millipore, catalog number: SX0741 )
- 0.1 M sulfuric acid (H2SO4)
- 0.2 N sodium hydroxide (NaOH)
- FeSO4·7H2O (VWR, catalog number: 97061-538 )
- 1 g/L ferrozine (Thermo Fisher Scientific, Fisher Scientific, catalog number: AC410570050 )
- 50 mM HEPES (pH = 7.0) (Sigma-Aldrich, catalog number: H3375 )
- Hydroxylamine-HCl (VWR, BDH®, catalog number: BDH9236-500G )
- Bio-Rad Protein Assay Kit II (Bio-Rad Laboratories, catalog number: 5000002 )
- 10% (w/v) oxalic acid (VWR, BDH®, catalog number: BDH7336-1 )
Equipment
- Heavy-duty round carboy (VWR, catalog number: 10755-104 )
- Standard magnetic stirrer
- Fermentor (Eppendorf, BrunswickTM, model: BioFlo®/Celligen® 115 )
- pH meter
- Plate centrifuge
- Autoclave
- Freezer
- Spectrophotometer
Software
- Eppendorf BioFlo®/Celligen® 115 fermentor automatic control software
- Adobe® Photoshop® software
Procedure
- Sediments are collected from the bottom of pools along the anoxic Fe(II)-rich artesian AMD flow paths. Sediments are collected downstream of the artesian discharges where the AMD have become well aerated (Figure 1A). Sediments are collected by carefully cutting and prying out intact pieces from the top 2 cm of the stream bed (Figure 1B). These samples are preserved in sterile plastic containers closed with lids, transported to the lab with dry ice, and stored in the 4 °C refrigerator for no longer than one week before use.
- Water is collected for microbial enrichments (Figure 1C). Water is collected from the anoxic artesian springs into acid washed plastic containers (completely filled with little or no headspace). Polypropylene carboys ranging in size from 12-40 L are used to transport the water to the lab. Immediately upon arrival to the lab, all water is filtered (0.45 μm sterile bottle-top filters) into plastic containers, purged with N2, wrapped in Al foil, and stored at 4 °C. Water is stored for no longer than one week before use.

Figure 1. Photographs of a typical Fe(II)-rich artesian AMD site (A), sediment collection (B) and water collection (C) - To develop enrichment cultures, 100 g of moist sediment is mixed with 1 L of 0.1% (m/v) sodium pyrophosphate (adjusted to pH of natural AMD water with sulfuric acid) in a sterile bottle (Figure 2A).
- For 30 min, this mixture is stirred at 400 rpm on a magnetic stirrer to separate cells from the sediment.
- The suspension is allowed to settle for 30 min and then 900 ml of the cell-containing supernatant is poured into a sterile 3 L chemostat reactor vessel (Eppendorf BioFlo®/Celligen® 115 fermentor) (Figure 2B). The reactor vessel is able to accommodate the low pH values (2 < pH < 4).
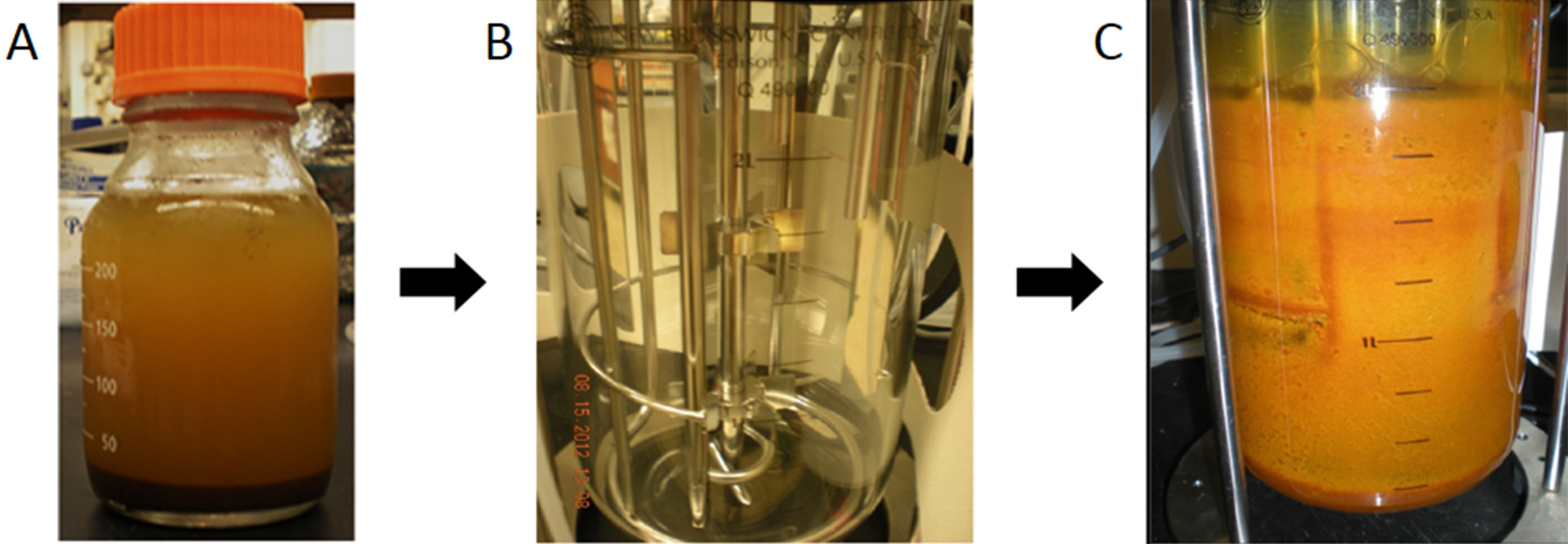
Figure 2. Photographs of enriching natural-occurring sediment microbes in a sterile chemostat reactor vessel. A. Cell separation step; B. Bioreactor vessel; C. Bioreactor in use. - The volume of the liquid in the chemostat reactor vessel is increased to 2 L by adding filtered (0.45-μm) site AMD water (Figure 2C).
- The chemostat is then operated in a no-flow, fed-batch mode for 4 to 6 weeks. Automated control components of the bioreactors maintained a constant pH, temperature and mixing speed, and continuously recorded dissolved oxygen. Feedback controls between the pH meter in the reactor and two peristaltic pumps delivering either 0.1 M H2SO4 or 0.2 N NaOH are used to maintain any desired pH set-point (Figure 3). A one-pass, tap water-fed, cooling coil within the reactor and a thermal jacket around the reactor are used to maintain the reactor temperature. Air is continuously pumped into the bottom of the reactor vessel to assure unlimited dissolved oxygen. During the enrichment period the pH set-point of the chemostat is same to the field water, the stirring rate is 50 rpm, the temperature is 20 °C and dissolved oxygen is unlimited (e.g., > 3 mg/L) (Sheng et al., 2016).
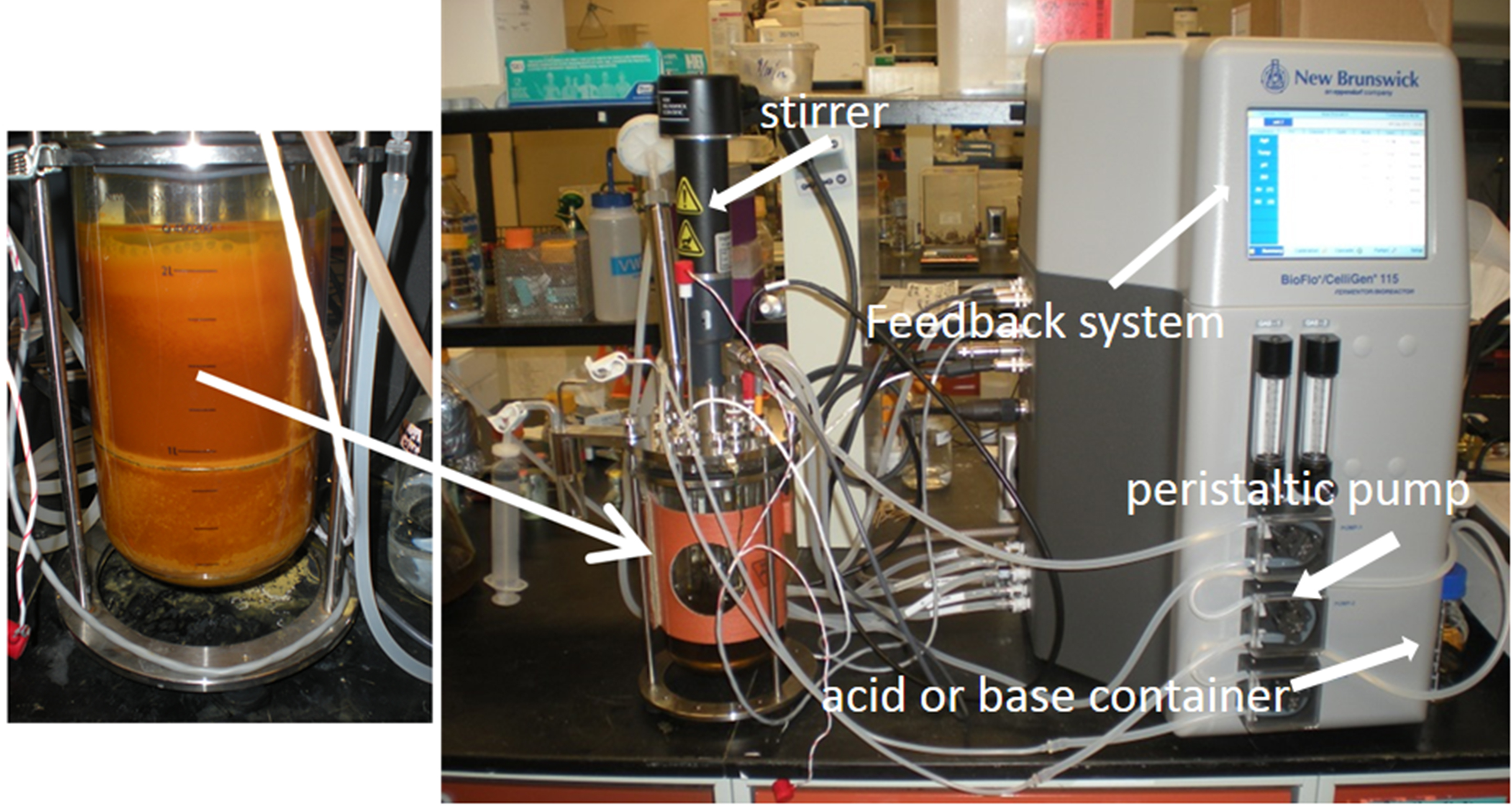
Figure 3. Photograph of bioreactor system including feedback system, peristaltic pumps and stirrer - Ferrous sulfate (FeSO4·7H2O) is discontinuously added to the reactor as the primary substrate to enrich for autotrophic Fe(II)-oxidizing microbes. Ferrous sulfate is added to yield 300 mg L-1 dissolved [Fe(II)] and added whenever the dissolved [Fe(II)] decreases below 30 mg L-1.
- After Fe(II) oxidation and total Fe removal rate have reached a pseudo-steady state condition (within ± 5% standard deviation), biofilms containing abundant Fe(II)-oxidizing microbial communities are clearly and evenly attached to the reactor wall (Figure 2C). After less than a month, the enrichment cultures required daily doses of Fe(II) because the microorganisms are oxidizing the Fe(II) at a fast rate.
- Sampling
- Samples for Fe(II) measurement are collected at the top of the liquid level inside the reactor vessel using sterile pipette. An unused port in the top of the vessel closed with a screw cap can be opened to take the sample. A total of 2 ml water is collected each time for Fe(II) and total Fe measurement. Dissolved Fe(II) and dissolved total Fe(T) (after reduction by hydroxylamine-HCl) are determined using the ferrozine assay (1 g/L ferrozine in 50 mM HEPES [pH 7.0]) with samples preserved with 0.1 N HCl (Stookey, 1970). The frequency of the sampling depends on the rates of Fe(II) oxidation. The bioreactor has to be sampled daily for the first two weeks and less often after.
- Biofilm samples are collected from the reactor wall and water samples are collected from the suspension to determine the biomass concentrations. The lid of the reactor vessel is opened and water is poured into a sterile container. After using sterile pipet to scrape the biofilm from the reactor wall, the water is poured back to the reactor vessel with closed lid. Biomass concentrations are determined based on protein (Bio-Rad Protein Assay Kit that uses Coomassie® Brilliant Blue G-250 dye). An area of at least 1 cm2 of biofilm is scraped from the wall of the reactor to use for the protein assay. A ruler is used as reference substance (Figure 4). Photographs of the scraped zone are used with Adobe® Photoshop® software to determine the exact area that is removed each time.
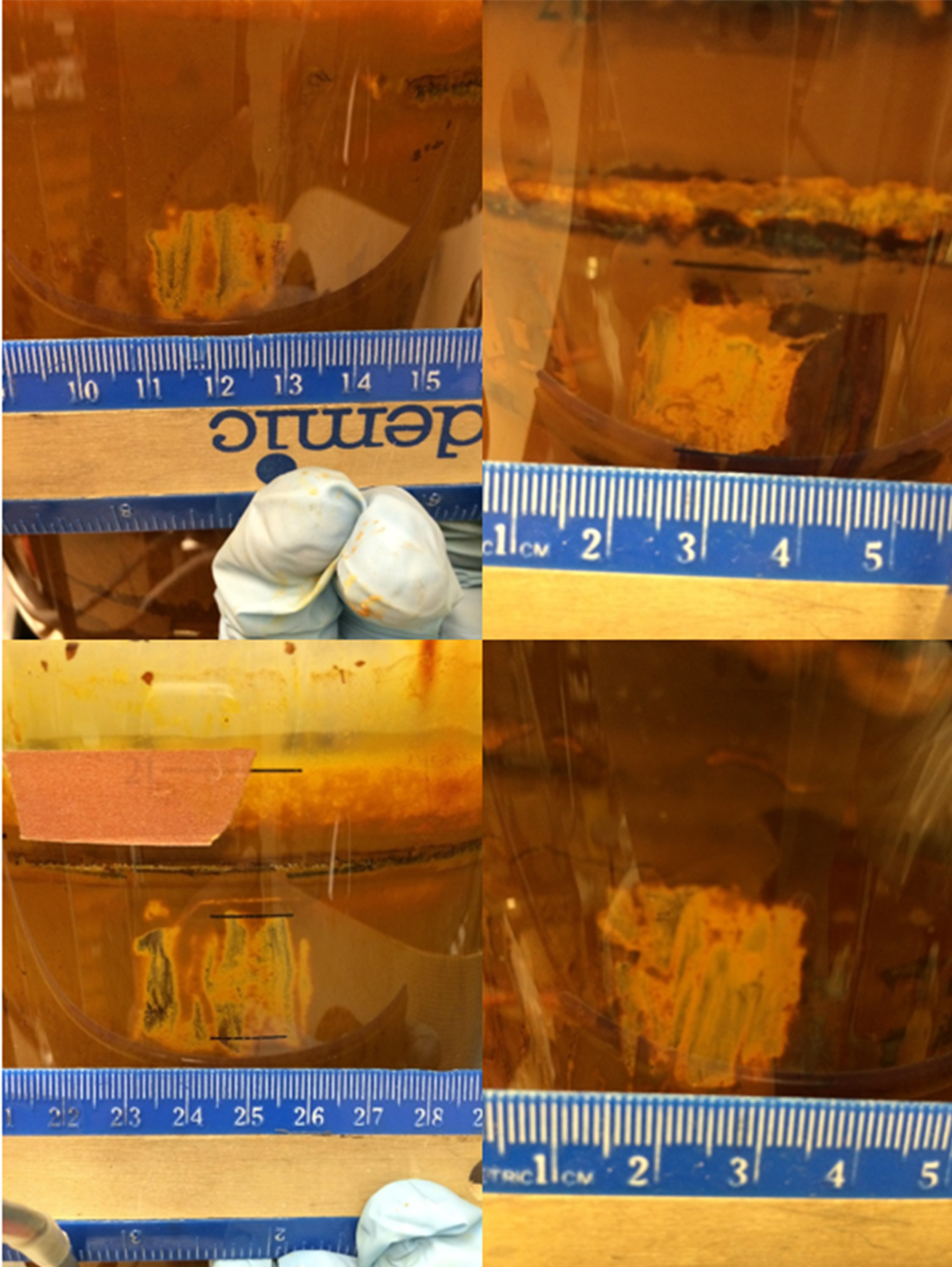
Figure 4. Exemplary Photographs of the scraped biofilm using a ruler as reference substance for area calculation - The collected biofilm is dissolved in 3 ml of 10% (w/v) oxalic acid. Once dissolved, the cells are lysed by placing 1 ml of this mix with 2 ml of 0.2 N NaOH, and then performing two freeze-thaw cycles on the sample in a -20 °C freezer for at least 6 h and 37 °C water bath for about 10-15 min. The protein colorimetric assay is then performed using the mixture containing the lysed cells.
- For the suspension, 135 ml of reactor liquid is centrifuged (13,000 x g for 10 min) to pelletize the cells. The masses of the Falcon® tubes used for centrifugation are measured before and after to determine the mass of each pellet. The liquid is then decanted and the cell pellet is analyzed exactly as the scraped cells described above.
- Samples for Fe(II) measurement are collected at the top of the liquid level inside the reactor vessel using sterile pipette. An unused port in the top of the vessel closed with a screw cap can be opened to take the sample. A total of 2 ml water is collected each time for Fe(II) and total Fe measurement. Dissolved Fe(II) and dissolved total Fe(T) (after reduction by hydroxylamine-HCl) are determined using the ferrozine assay (1 g/L ferrozine in 50 mM HEPES [pH 7.0]) with samples preserved with 0.1 N HCl (Stookey, 1970). The frequency of the sampling depends on the rates of Fe(II) oxidation. The bioreactor has to be sampled daily for the first two weeks and less often after.
Data analysis
- Assuming that the chemostat operates as a completely-mixed reactor, zero-order rates of Fe(II) oxidation [mol Fe(II) L-1 sec-1] in the chemostat are calculated as:
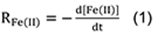
Where,
d[Fe(II)] is decreased dissolved Fe(II) concentration (mol L-1),
dt is increased incubation time. - For the enrichment experiment, the system reaches a pseudo-steady state with respect to the Fe(II) oxidation rate. In order to deem certain measured points within the pseudo-steady state and others not, a range of plus and minus 5% standard deviation of the last measurement is calculated. Any points that fall within the plus or minus 5% standard deviation range are deemed to be at pseudo-steady state. This statistical method placed emphasis on the last measured point since all other points are compared to it.
- To determine the concentration of cells in suspension and in the biofilm, a Bio-Rad protein assay kit is used. Once the protein concentrations are obtained, the derived conversion factor of 0.15 x 10-12 g protein/typical cell is used to convert the mass of protein present to the number of cells.
Notes
- Because enriched Fe(II)-oxidizing microbial communities are greatly influenced by pH and Fe(II) concentrations (Sheng et al., 2016), the pH set-point and Fe(II) concentrations of the chemostat can be adjusted according to the pH and Fe(II) concentrations of field AMD.
- Sediments and water are collected for microbial enrichments. Sediments are collected from the bottom of pools along the anoxic Fe(II)-rich artesian AMD flow paths. Water is collected from the anoxic artesian springs. To develop enrichment cultures, 100 g of moist sediment is mixed with 1 L of 0.1% (m/v) sodium pyrophosphate (adjusted to pH of natural AMD water with sulfuric acid).
- Chemostat bioreactors are operated at pH of the field AMD, temperature 20 °C, stirring rate 50 rpm and unlimited dissolved oxygen (> 3 mg/L) for 4-6 weeks.
- Ferrozine assay (1 g/L ferrozine in 50 mM HEPES [pH 7.0]) is used to measure Fe(II) oxidation rate (Stookey, 1970).
- Attached-growth and suspended biomass is dissolved in 10% (w/v) oxalic acid, mix with 0.2 N NaOH, and then performing two freeze-thaw cycles to lyse cells. Biomass concentrations are determined based on protein (Bio-Rad Protein Assay Kit that uses Coomassie® Brilliant Blue G-250 dye).
Recipes
- Solution for Ferrozine assay (pH 7.0)
1 g/L ferrozine
50 mM HEPES - Enrichment of acidophilic bacteria
100 g of moist sediment is mixed with 1 L of 0.1% (m/v) sodium pyrophosphate
Adjusted to pH of natural AMD water with sulfuric acid
Acknowledgments
This protocol is adapted from Sheng et al. (2016). This work is partially supported by the US Office of Surface Mining Reclamation and Enforcement under Cooperative Agreement S11AC20005, by the China Scholarship Council (to Y.S.), and by the Appalachian Research Initiative for Environmental Science (ARIES). ARIES is an industrial affiliates program at Virginia Tech, supported by members that include companies in the energy sector. The opinions and recommendations expressed herein are solely those of the authors and do not imply any endorsement by ARIES.
References
- Brown, J. F., Jones, D. S., Mills, D. B., Macalady, J. L. and Burgos, W. D. (2011). Application of a depositional facies model to an acid mine drainage site. Appl Environ Microbiol 77(2): 545-554.
- DeSa, T., Brown, J. and Burgos, W. (2010). Laboratory and field-scale evaluation of low-pH Fe(II) oxidation at Hughes Borehole, Portage, Pennsylvania. Mine Water Environ 29 (4):239-247.
- Hedrich, S. and Johnson, D. B. (2012). A modular continuous flow reactor system for the selective bio-oxidation of iron and precipitation of schwertmannite from mine-impacted waters. Bioresour Technol 106: 44-49.
- Heinzel, E., Hedrich, S., Janneck, E., Glombitza, F., Seifert, J. and Schlomann, M. (2009a). Bacterial diversity in a mine water treatment plant. Appl Environ Microbiol 75(3): 858-861.
- Heinzel, E., Janneck, E., Glombitza, F., Schlomann, M. and Seifert, J. (2009b). Population dynamics of iron-oxidizing communities in pilot plants for the treatment of acid mine waters. Environ Sci Technol 43(16): 6138-6144
- Janneck, E., Arnold, I., Koch, T., Meyer, J., Burghardt, D. and Ehinger, S. (2010). Microbial synthesis of schwertmannite from lignite mine water and its utilization for removal of arsenic from mine waters and for production of iron pigments. In: Wolkersdorfer, C. and Freund, A. (Eds.). Mine water and innovative thinking. Proceedings of the international mine water association symposium 131-134.
- Larson, L. N., Sánchez-España, J. and Burgos, W. (2014a). Rates of low-pH biological Fe(II) oxidation in the appalachian bituminous coal basin and the Iberian pyrite belt. Appl Geochem 47:85-98.
- Larson, L. N., Sánchez-España, J., Kaley, B., Sheng, Y., Bibby, K. and Burgos, W. D. (2014b). Thermodynamic controls on the kinetics of microbial low-pH Fe(II) oxidation. Environ Sci Technol 48(16): 9246-9254.
- Tischler, J. S., Wiacek, C., Janneck, E. and Schlömann, M. (2013). Microbial abundance in the schwertmannite formed in a mine water treatment plant. Mine Water Environ 32:258-265.
- Sheng, Y., Bibby, K., Grettenberger, C., Kaley, B., Macalady, J. L., Wang, G. and Burgos, W. D. (2016). Geochemical and temporal influences on the enrichment of acidophilic iron-oxidizing bacterial communities. Appl Environ Microbiol 82(12): 3611-3621.
- Stookey, L. L. (1970). Ferrozine--a new spectrophotometric reagent for iron. Anal Chem 42(7):779-781.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sheng, Y., Kaley, B. and Burgos, W. D. (2017). Enriching Acidophilic Fe(II)-oxidizing Bacteria in No-flow, Fed-batch Systems. Bio-protocol 7(3): e2130. DOI: 10.21769/BioProtoc.2130.
Category
Microbiology > Microbial physiology > Adaptation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










