- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assay to Access Anthelmintic Activity of Small Molecule Drugs Using Caenohabidtis elegans as a Model
Published: Vol 7, Iss 2, Jan 20, 2017 DOI: 10.21769/BioProtoc.2113 Views: 11871
Reviewed by: Neelanjan BoseAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
![Photoaffinity Labeling of Respiratory Complex I in Bovine Heart Submitochondrial Particles by Photoreactive [<sup>125</sup>I] amilorides](https://en-cdn.bio-protocol.org/imageup/arcimg/20190902091054730.jpg?t=1771617229)
Photoaffinity Labeling of Respiratory Complex I in Bovine Heart Submitochondrial Particles by Photoreactive [125I] amilorides
Masatoshi Murai and Hideto Miyoshi
Sep 5, 2019 4778 Views
Abstract
This protocol proposes to use the nematode Caenorhabditis elegans as a model to screen and study the anthelmintic activity of natural and synthetic compounds and to observe their effects on the morphology and the ultrastructure of the helminths. Furthermore, C. elegans can be used to investigate the anthelmintic activity in embryonated eggs, larval stages and in the adults’ survival. As most current anthelmintics are not effective against all nematode life stages, this protocol can contribute to the identification of new alternatives to helminthic infections (Sant’Anna et al., 2016).
Keywords: C. elegansBackground
Caenorhabditis elegans is a model organism for parasite nematode research and an excellent system for the screening of compounds with potential anthelmintic activity, because it is inexpensive, readily available, and easy to work with. In addition, the use of C. elegans in assays to investigate nematode behavior, locomotion, reproduction and death is uncomplicated and reliable (Simpkin and Coles, 1981). The protocols for screening new compounds on C. elegans were first carried out in axenic liquid medium in deep well microscope slides (Tomlinson et al., 1985) or using the drugs added to melted modified NGM agar (Driscoll et al., 1989). These methods are not effective in drug screening as axenic cultures, containing low food supply, trigger the intra-uterine birth causing maternal death (endotokia matricida) (Lenaerts et al., 2008) and drugs added to melted agar can modify drug stability due to the high temperatures. In this protocol, we used 96-well plates with liquid medium supplied with Escherichia coli to evaluate each stage (eggs, L1-L2 larvae, L3-L4 larvae and adults) independently.
Materials and Reagents
- Transfer pipette
- 15 ml Falcon tubes (Corning, Falcon®, catalog number: 352095 )
- 96-well plate, flat bottom, polystyrene, 0.32 cm2, sterile. TPP tissue culture plates (Sigma-Aldrich, catalog number: Z707910 )
- Tissue culture dishes of polystyrene TPP- diam. 60 x 15 mm, surface area size 22.1 cm2 with NGM (Sigma-Aldrich, catalog number: Z707678 )
- C. elegans N2 strain
- Escherichia coli OP50 strain
- Drugs to screen
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 795429 )
- Hypochlorite (NaClO) (Sigma-Aldrich, catalog number: 13440 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S5136 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Magnesium sulfate heptahydrate (MgSO4·7H2O), BioUltra ≥ 99.5% (KT) (Sigma-Aldrich, catalog number: 63138 )
- Potassium phosphate dibasic (K2HPO4), ACS reagent, ≥ 98% (Sigma-Aldrich, catalog number: P3786 )
- Cholesterol (Sigma-Aldrich, catalog number: C3045 )
- Ethanol (p.a., without additive, ≥ 99.8%) (Sigma-Aldrich, catalog number: 24102 )
Note: This product has been discontinued. - Citric acid monohydrate (ACS reagent, ≥ 99.0%) (Sigma-Aldrich, catalog number: C1909 )
- Tri-potassium citrate monohydrate (Sigma-Aldrich, catalog number: 6100-05-6 )
- Disodium EDTA (98.5-101.5%, BioUltra) (Sigma-Aldrich, catalog number: E1644 )
- Iron (II) sulfate heptahydrate (FeSO4·7H2O) (Sigma-Aldrich, catalog number: 215422 )
- Manganese(II) chloride tetrahydrate (MnCl2·4H2O) (Sigma-Aldrich, catalog number: 203734 )
- Zinc sulfate heptahydrate (ZnSO4·7H2O) (BioReagent, suitable for cell culture) (Sigma-Aldrich, catalog number: 7446-20-0 )
- Copper(II) sulfate pentahydrate (CuSO4·5H2O) (BioReagent, suitable for cell culture, ≥ 98%) (Sigma-Aldrich, catalog number: C8027 )
- Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C1016 )
- Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: M7506 )
- Lysing solution (see Recipes)
- M9 buffer (1 L) (see Recipes)
- S medium (1 L) (see Recipes)
Equipment
- Clinical centrifuge (Thermo Fisher Scientific, catalog number: 22-029-416 )
- Inverted microscope (ZEISS, model: Axio Vert.A1 )
- Biochemical oxygen demand (BOD) incubator (Thermo Fisher Scientific, Fisher ScientificTM, catalog number: 37-20 )
- Micropipet, 100-1,000 μl volume (Nichipet Eco pipette, catalog number: Z710199 )
- Autoclave
Procedure
- Culture synchronization (adapted from Stiernagle, 2006)
- Begin the procedure with C. elegans plates containing nearly 500 gravid hermaphrodites. Add 5 ml of M9 buffer to the plate and gently stir the liquid with a pipette to dislodge the worms from the agar.
- Using a pipette, transfer the worms to a 15 ml sterile Falcon tube, centrifuge at 800 x g for 1 min. The worm pellet must be suspended in 3.5 ml total volume.
- Add 1.5 ml of lysing solution to the tube. Shake the tube gently for 5 min, manually, looking under a microscope to check if worms have disintegrated or not. Most of the worm bodies have dissolved, centrifuge at 800 x g for 1 min.
- Remove most of lysing solution without disturbing the egg pellet.
- Then, suspend the pellet in 5 ml of M9 buffer, centrifuge 1,000 x g for 5 min, remove the supernatant, add fresh M9 and repeat these steps 2 more times.
- The eggs obtained can be conducted in three different procedures in parallel.
I, The eggs can be incubated with the drugs directly for 15 h at 20 °C.
II, The eggs can be incubated at 20 °C for 15 h to produce larvae, which can be incubated at maximum for 24 h with the drugs, to avoid development in advanced stages.
III, The eggs can be incubated at 20 °C for 3 days to produce adults. Adults can be incubated, at maximum, for 3 days with the drugs to avoid new adult generations. Figure 1 summarizes the culture synchronization and the use of the three different life stages of C. elegans – embryonic eggs, larvae and adults (steps B, C and D) to evaluate anthelmintic activity of drugs.
Figure 1. Scheme summarizing the culture synchronization procedure and the use of, embryonic eggs, larvae and the adults (steps B, C and D) of Caenorhabditis elegans to evaluate anthelmintic activity of drugs
- Egg hatch assay
- After the synchronization procedure, transfer the eggs to a 96-well plate with a pipette.
- Add approximately 30 eggs per well in 200 µl of S medium.
- Count and identify the embryonic stages inside the eggs with an inverted microscope to observe the larval development.
Note: The presence of the gastrula form is important to determine the initial pattern before treatment with the drugs and to verify if the lysing solution affected or not the eggs. The gastrula form can be identified according to Figure 2B. - Add the different concentrations of drugs and incubate for 15 h at 20 °C in a BOD incubator.
- At the end of the incubation, the percentage of hatched and unhatched eggs and the L1 larvae will be determined for each of the different drug concentrations by light microscopy. Triplicates of six independent experiments should be performed.
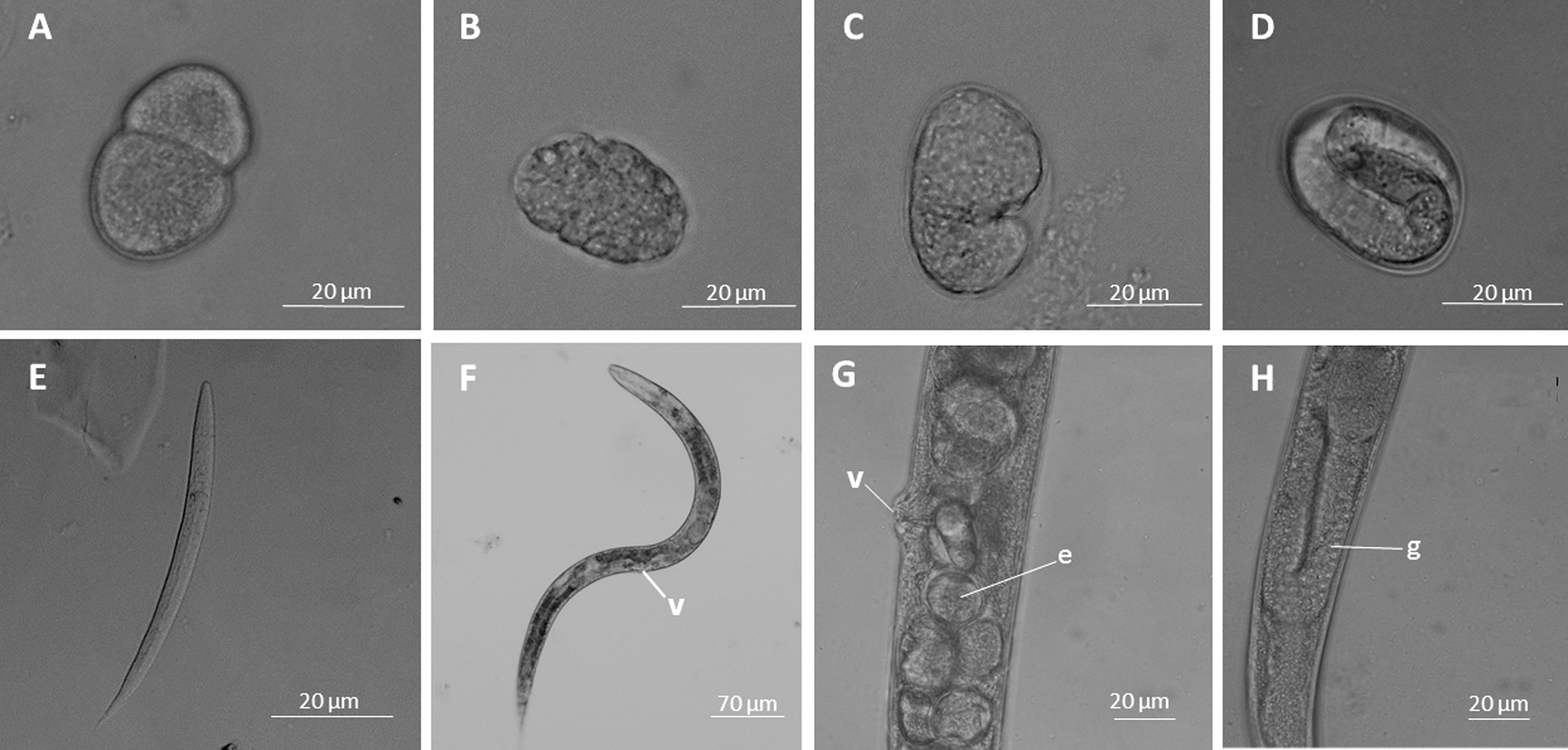
Figure 2. Phase contrast light microscopy images showing different C. elegans stages. A. Embryo in the first cleavage; B. Gastrula form; C. Embryo with comma form; D. Egg with a larva inside (the 3-fold stage indicates the complete larval development); E. L1 stage; F. Hermaphrodite, the vulva (v) can be observed; G. Vulva (v) and numerous eggs (e) in higher magnification; H. The hermaphrodite gonad (g) can be observed.
- Larval development assay
- After the synchronization procedure, put the eggs in microtubes containing S medium and E. coli under gentle agitation, for 15 h at 20 °C at the BOD incubator.
- After this time, collect the larvae at the first stages (L1/L2) (Figure 2E). Alternatively, the eggs can hatch in the absence of food as development will be arrested and larvae will stay as L1.
- Adjust the concentration of larvae to 20 larvae/50 μl in S medium.
- Incubate for 24 h at 20 °C in a 96- well plates containing S medium supplemented with E. coli and the different concentrations of the drugs to be analyzed. Triplicates of six independent experiments should be performed.
- Assays using adults
- After the synchronization procedure, transfer the eggs to NGM (Nematode growth medium) plates seeded with E. coli with a pipette. The eggs were incubated at 20 °C for three days in the BOD incubator to obtain most of the adult nematodes at the same age.
- Collect the adult worms with a transfer pipette by washing the NGM plates with 5 ml of M9 to dislodge the worms and centrifuge at 800 x g for 5 min in a Falcon tube. Remove the supernatant and wash the pellet in the same buffer (three times).
- Place thirty nematodes per well in a 96-well plate containing S medium 200 µl supplemented with E. coli. The bacteria can be autoclaved to avoid excessive growth during the period of incubation. We suggest 2 x 103 bacteria/ml approximately.
- Add the different concentrations of the drugs to be screened in the study. The drugs must be omitted in the wells used as negative controls.
- Incubate at 20 °C for 3 days in the BOD incubator. Three days should be the maximum period of incubation to avoid the presence of new generations of adults.
- After this period, the survival was evaluated by counting the live and dead worms by light microscopy considering the motility and the paralysis of the pharyngeal bulb and the total loss of motility with the occurrence of straight bodies as shown in Video 1. Use an inverted microscope to make these observations. Triplicates of six independent experiments should be performed.Video 1. Movie illustrating the motility and the paralysis of the pharyngeal bulb by phase contrast light microscopy with the occurrence of straight bodies of dead worms
Data analysis
- To evaluate the survival of adults and larvae and their motility, living worms must be counted by optical microscopy excluding the larvae stages, which hatched during the assay. The size and the presence of reproductive organs are used as criteria to follow the original adult population (Figures 2F-2H).
- In the egg hatch assay, the percentage of hatched and unhatched eggs and survival of L1 larvae should be determined for each different drug concentration (as in Sant’Anna et al., 2016).
- Kaplan Meyer tests can be used to analyze the survival of individuals along the treatment and the survival curves should be compared by the log rank test (Kaplan and Meier, 1958).
Notes
The absence or low concentration of cholesterol affects the embryos survival, because it is essential for the development of the oocytes. The S medium should contain adequate concentrations of cholesterol (Greenstein, 2005).
Recipes
- Lysing solution
5 N NaOH
1% hypochlorite
Prepared fresh for each batch - M9 buffer (1 L)
3 g KH2PO4
6 g Na2HPO4
5 g NaCl
0.25 g MgSO4·7H2O
Autoclave and stock at 4 °C - S medium (1 L)
- S basal
5.85 g NaCl
1 g K2HPO4
6 g KH2PO4
1 ml cholesterol (5 mg/ml in ethanol) - 10 ml 1 M potassium citrate, pH 6
20 g citric acid monohydrate
293.5 g tri-potassium citrate monohydrate
Add H2O to 1 L
Sterilize by autoclaving - 10 ml trace metals solution
1.86 g disodium EDTA
0.69 g FeSO4·7H2O
0.2 g MnCl2·4H2O
0.29 g ZnSO4·7H2O
0.025 g CuSO4·5H2O
Add H2O to 1 L
Sterilize by autoclaving and keep protected from light - 3 ml 1 M CaCl2
- 3 ml 1 M MgSO4
Note: Manipulate the components under sterile conditions; do not autoclave the complete medium. All the recipes were adapted from Stiernagle (2006). - S basal
Acknowledgments
The authors thank the Caenorhabditis Genetics Center for donating the C. elegans wild type and the Brazilian agencies: Fundação de Coordenação de aperfeiçoamento de Pessoal de Nível Superior-CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ for supporting this work.
References
- Driscoll, M., Dean, E., Reilly, E., Bergholz, E. and Chalfie, M. (1989). Genetic and molecular analysis of a Caenorhabditis elegans β-tubulin that conveys benzimidazole sensitivity. J Cell Biol 109(6 Pt 1): 2993-3003.
- Greenstein, D. (2005). Control of oocyte meiotic maturation and fertilization. WormBook: 1-12.
- Kaplan, E. L. and Meier, P. (1958). Nonparametric estimation from incomplete observations. J Amer Statist Assn 53(282): 457-481.
- Lenaerts, I., Walker, G. A., Van Hoorebeke, L., Gems, D. and Vanfleteren, J. R. (2008). Dietary restriction of Caenorhabditis elegans by axenic culture reflects nutritional requirement for constituents provided by metabolically active microbes. J Gerontol A Biol Sci Med Sci 63(3): 242-252.
- Sant’Anna, V., de Souza, W. and Vommaro, R. C. (2016). Anthelmintic effect of herbicidal dinitroanilines on the nematode model Caenorhabditis elegans. Exp Parasitol 167: 43-49.
- Simpkin, K. G. and Coles, G. C. (1981). The use of Caenorhabditis elegans for anthelmintic screening. J Chem Technol Biotechnol 31(1): 66-69.
- Stiernagle, T. (2006). Maintenance of C. elegans. WormBook 2(11).
- Tomlinson, G., Albuquerque, C. A. and Woods, R. A. (1985). The effects of amidantel (BAY d 8815) and its deacylated derivative (BAY d 9216) on Caenorhabditis elegans. Eur J Pharmacol 113(2): 255-262.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sant’Anna, V., de Souza, W. and Vommaro, R. C. (2017). Assay to Access Anthelmintic Activity of Small Molecule Drugs Using Caenohabidtis elegans as a Model. Bio-protocol 7(2): e2113. DOI: 10.21769/BioProtoc.2113.
Category
Biochemistry > Other compound > Small molecule drugs
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link













