- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Primary Culture of Mouse Neurons from the Spinal Cord Dorsal Horn
Published: Vol 7, Iss 1, Jan 5, 2017 DOI: 10.21769/BioProtoc.2098 Views: 17339
Reviewed by: Jia LiGeoff LauFabiana Scornik

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Primary afferents of sensory neurons mainly terminate in the spinal cord dorsal horn, which has an important role in the integration and modulation of sensory-related signals. Primary culture of mouse spinal dorsal horn neuron (SDHN) is useful for studying signal transmission from peripheral nervous system to the brain, as well as for developing cellular disease models, such as pain and itch. Because of the specific features of SDHN, it is necessary to establish a reliable culture method that is suitable for testing neural response to various external stimuli in vitro.
Keywords: NeuronBackground
Unlike existing protocols for culturing isolated mice primary neurons from hippocampus or cerebral cortex, few methods of culturing SDHN in vitro have been reported. This protocol was mainly based on previously described methods (Hu et al., 2003; Hugel and Schlichter, 2000). Here we made a few modifications including reagents, recipes, dissection and described step-by-step procedures of the dissection and culture of primary SDHN from newborn mice. In this protocol, neurons were gained using the enzymatic (papain) digestion method from fresh spinal dorsal horn tissues directly. The culture of SDHN in vitro can be used for further experiments, such as electrophysiological recordings, immunocytochemistry, and Ca2+ imaging, which better support cell behaviors in the spinal cord.
Materials and Reagents
- Coverslips (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 174950 )
- 24-well cell culture plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 142475 )
- Cell culture dishes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 153066 )
- Sterile centrifuge tubes (5 ml and 50 ml)
- Cotton ball
- Pipette tip
- Cell strainer (40 μm) (Corning, Falcon®, catalog number: 352340 )
- Ice
- 3 days-old mice
- Diethyl ether (Sigma-Aldrich, catalog number: 346136 )
- 75% ethanol
- Cytosine arabinoside (Sigma-Aldrich, catalog number: C6645 )
- 4% formaldehyde
- 5% goat serum
- MAP2 antibody (Sigma-Aldrich, catalog number: M1406 )
- Anti-mouse FITC-conjugated secondary antibody
- DAPI (Sigma-Aldrich, catalog number: M9542 )
Note: This product has been discontinued. - Collagen (Sigma-Aldrich, catalog number: C7661 )
- Poly-D-lysine (Sigma-Aldrich, catalog number: P7405 )
- HEPES (1 M) (Thermo Fisher Scientific, GibcoTM, catalog number: 15630080 )
- HBSS (Thermo Fisher Scientific, GibcoTM, catalog number: 14025092 )
- Papain (Worthington Biochemical, catalog number: LS003119 )
- Neuro basal (Thermo Fisher Scientific, GibcoTM, catalog number: 10888022 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 1099141 )
- Heat-inactivated horse serum (Thermo Fisher Scientific, GibcoTM, catalog number: 26050070 )
- B27 (50x) (Thermo Fisher Scientific, GibcoTM, catalog number: 17504044 )
- Glutamax (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- Penicillin/Streptomycin (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Collagen stock solution (see Recipes)
- Poly-D-lysine (PDL) stock solution (see Recipes)
- Coating solution (see Recipes)
- HBSS + HEPES solution (see Recipes)
- Papain solution (see Recipes)
- Culture media (see Recipes)
Equipment
- Surgical scissors (decapitation/dissecting spinal column from body) (RWD Life Science, catalog number: S14014-14 )
- Blunt-tipped forceps (dissecting/holding spinal column) (RWD Life Science, catalog number: F13017-12 )
- Long and narrow-tipped spring scissors (dissecting spinal cord/cutting nerve roots) (66 Vision, catalog number: 54053B )
- Small-tipped spring scissors (cutting dura) (Fine Science Tools, catalog number: 15000-02 )
- Microforceps (holding spinal cord/trimming off dura) (World Precision Instruments, catalog number: 14098 )
- Scalpel #15 sterilized surgical blade (cutting spinal cord to isolate dorsal horn) (Shanghai Jinhuan Medical, catalog number: YY0174-2005 )
- Tissue culture hood (Suzhou Antai Airtech, model: SW-CJ-1F(D) )
- Stereomicroscope (OLYMPUS, model: SZ61 )
- Benchtop centrifuge (4 °C, 50 ml tube) (Eppendorf, model: 5430R )
- Pipette (Eppendorf, model: ES-1000 )
- Haemocytometer (Shanghai anxin optical instrument manufacture, model: XB-K-25 )
- Stackable CO2 incubator (Eppendorf, model: Galaxy®170R )
Procedure
- Select three 3 days-old mice.
- Coat coverslips with PDL/collagen/water coating solution.
- Put coverslips in a 24-well cell culture plate, with 1 piece/well. Add 500 μl coating solution in each well. This process is done under the hood.
- Incubate for 1-3 h in an incubator (37 °C).
- Transfer to the hood with cover off for 30 min to 1 h.
- Discard the excess solution, then let the coverslips air dry in the hood.
- Preparation (this process is done outside the hood in cell culture room)
- Place 5 small culture dishes (35 mm) filled with 4 ml ice-cold HBSS + HEPES solution on ice for dorsal horn tissue isolation.
- Prepare about 20 ml ice-cold HBSS + HEPES solution on ice for cell washes later.
- Preheat papain solution in 5 ml tube in the incubator for tissue digestion later.
- Preheat 20 ml aliquot of culture media in the incubator.
- Spinal cord dorsal horn dissection (Video 1, this process is done outside the hood in cell culture room). Add 1 ml Diethyl ether onto a cotton ball and put it into a 50 ml tube. Put the animal into the tube. Take it out after the animal stops moving. Spray the animal with 75% ethanol, decapitate with surgical scissors under deep diethyl ether anesthesia.Video 1. Dissection of the spinal cord dorsal horn
- Cut the skin on the back and isolate the spinal column.
- Clamp the spinal column with blunt forceps.
- Pull up the column, cut the ribs lateral to the midline of spinal column and the ventral tissues attached to the column. Start rostrally and progress caudally.
- Make a transverse cut through the spinal column at the most caudal end and free the entire spinal column.
- Place the dissected spinal column into a culture dish with ice-cold HBSS + HEPES solution under the stereomicroscope. Arrange the column so that the ventral side is up and the rostral end is towards the right (Figure 1).
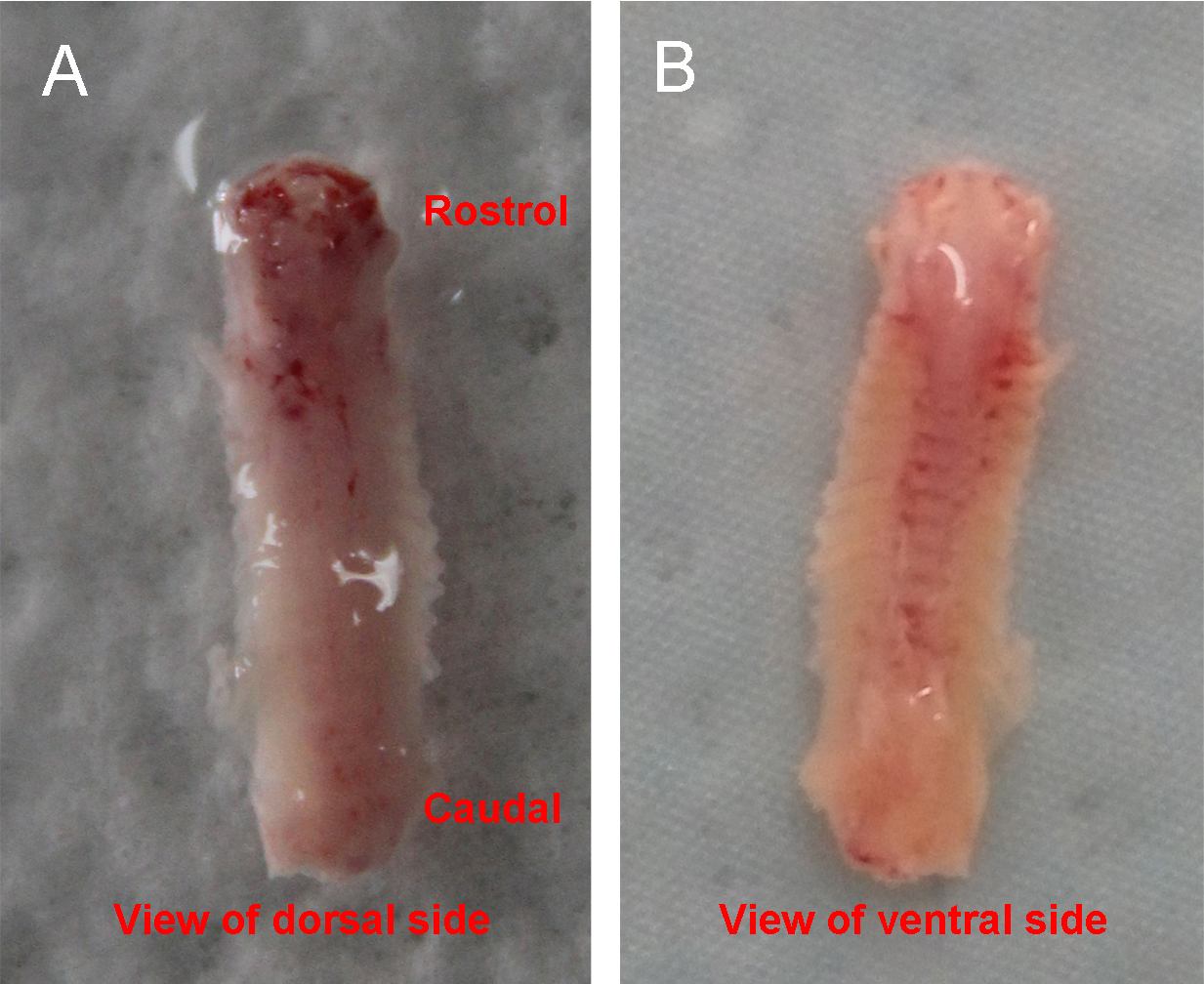
Figure 1. The isolated spinal column. A shows the view of dorsal side. There are more attached muscles than the ventral side. B shows the view of ventral side. Ribs are attached to the column. - Make ventral ‘laminectomy’
- Use large spring scissors to cut off the vertebral bones. Start from the rostral end and move towards the caudal end. Alternately cut the left side and the right side of the vertebrae. During the cutting, insert one blade of the scissors into the spinal canal and angle the scissors down and laterally away from the cord. Then use the tip of the scissors to lift up the ventral disk of the spinal column. Discard that chunk of vertebrae and the attached tissues (Figure 2).
- Repeat the procedure until the ventral surface of the spinal cord is exposed.

Figure 2. Make ventral ‘laminectomy’. A and B. Cut off the vertebral bones from rostral to caudal side. - Along the dorsolateral side of the cord, cut the connected nerve roots with large spring scissors. Always angle the scissors down and away from the cord (Figure 3). Gently free the cord from the spinal column with the closed tip of the spring scissors. Discard spinal column and other tissues.

Figure 3. Cut the attached nerve roots (A) and isolate the cord (B) - Place the cord into a new culture dish with ice-cold HBSS + HEPES solution. Put the rostral side of the cord forward (judge the caudal and rostral ends according to the direction of the nerve roots extended from the spinal cord) and the dorsal side is up (a blood vessel can be seen in the middle of the surface of the dorsal side) (Figure 4A).

Figure 4. Isolate and cut the spinal cord. A. A blood vessel (marked by red dotted line) can be seen in the middle of the surface of the dorsal side. The direction (yellow arrows) of the nerve roots extended from the spinal cord to the caudal end. B. Cut the spinal cord along the midline, so that the medial side faces up. C. Cut approximately ¼ of dorsal part of spinal cord from caudal to rostral. - Cut through the dura along the midline of the cord with small spring scissors.
- Use the closed tips of microforceps to gently press on the dorsal surface of the cord. (Make sure the cord is on the bottom of the dish, otherwise it will be difficult to manipulate and slice.)
- Cut from caudal end. While cutting, insert one blade (only the tip) of scissors under the dura and gently lift the dura to avoid destroying the cord.
- To cut ‘forward’ (rostral end), slide scissors rostrally while sliding microforceps caudally.
- Trim the dura attached to the dorsal surface of the cord with microforceps.
- Cut the spinal cord along midline (caudal-rostro axis) with scalpel (Figure 4B). Use the two tips of the microforceps to gently fix both sides of the cord and cut from the caudal to the rostral.
- Dissect the dorsal horn
- Take one half of the spinal cord and set the lateral side of the cord down and the medial side up (Figure 4B).
- Cut approximately ¼ of dorsal part of spinal cord from caudal to rostral.
- For the right hemisected cord, hold microforceps with your left hand and make careful cuts with scalpel held with right hand from caudal to rostral (Figure 4C).
- For the left hemisected cord, turn it around and cut from rostral to caudal.
- Discard the remaining ¾ of ventral spinal cord.
- Place dissected dorsal horn strip into a new culture dish with ice-cold HBSS + HEPES solution.
Note: Following steps are done in the hood.
- After collecting all the dorsal horn strips, transfer them to the 5 ml tube filled with the papain solution (step 3c).
- Digest for 30 min in the CO2 incubator at 37 °C (swirl every 5 or 10 min).
- Wash with 1-2 ml ice-cold HBSS + HEPES solution by aspirating liquid (do not aspirate the tissue). Put the tube stand for 2 min and let the tissue go down. Discard the liquid.
- Repeat washing twice.
- Wash the remaining tissue with 1 ml culture media.
- Discard as much liquid as possible and add another 1 ml culture media.
- Triturate for 6-7 times with a 1 ml fire-polished pipette tip (place the tip of pipette against wall of tube and aspirate the visible pieces up and down gently, avoid making any bubble) and then stand for 2-3 min.
- Collect about 600 μl supernatant and filter the collected supernatant with 40 μm cell strainer.
- Repeat steps 9-12 to collect about 3 ml in total and put the filtered supernatant into a 50 ml tube.
- Centrifuge at 1,000 x g for 5 min at 4 °C and remove as much supernatant as possible.
- Add 1 ml culture media to resuspend cells.
- Count cells using a hemacytometer and adjust the cell density to 5 x 105/ml with culture medium.
- Plate 200 μl supernatant onto the coated coverslips.
- Incubate for 60 min at 37 °C, then check for adherent cells and discard suspension cells, add 1 ml culture media to each well (for coverslips in 24-well plate).
- Change culture media on day 1 (replace half of the culture media), and then every 2 days (replace all).
- Two days after seeding, add cytosine arabinoside (10 μM, once) to the culture medium and maintain for 24 h to reduce glial proliferation.
- Use the cells for further experiments (Figure 5).
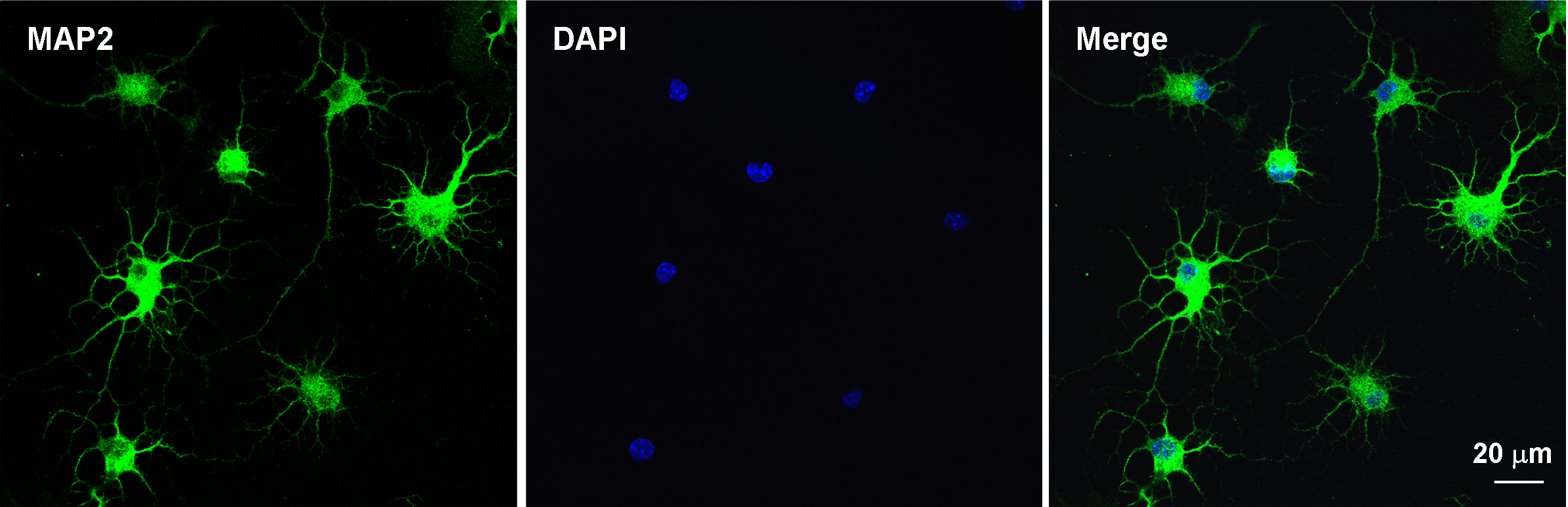
Figure 5. Immunocytochemistry for evaluating the purity of the primary cultures of spinal dorsal horn neuron after 7 days. Double staining of MAP2 (neuronal marker, green) and DAPI (nuclear marker, blue) shows that majority of cultured cells are neurons.
Data analysis
To evaluate the purity of the primary cultures of spinal dorsal horn neuron, the double staining of immunofluorescence is used. Seven-days culture cells are fixed with 4% formaldehyde for 20 min and blocked with 5% goat serum for 2 h at room temperature. Then incubate overnight at 4 °C with mouse anti-MAP2 antibody (1:500) and incubate for 1 h at room temperature with anti-mouse FITC-conjugated secondary antibody (1:1,000; Jackson ImmunoResearch). After immunostaining, add 4,6-diamidino-2-phenylindole (DAPI) (0.1 g/ml) in the culture plates for 5 min at room temperature to stain all the nuclei of cells (Jiang et al., 2016).
Recipes
- Collagen stock solution
Make up to 2 mg/ml in sterile double distilled water
Aliquot and store at 4 °C - Poly-D-lysine (PDL) stock solution
Make up to 0.1 mg/ml in sterile double distilled water
Aliquot and store at -20 °C - Coating solution
0.2 mg/ml collagen
10 μg/ml Poly-D-lysine
Dilute stock solutions (Recipes 1 and 2) with sterile double distilled water - HBSS + HEPES solution
Add 5 ml HEPES (1 M) to 500 ml HBSS and aliquot into 50 ml tubes - Papain solution
Add 45 μl papain to 3 ml HBSS + HEPES solution - Culture media
Neuro basal supplemented with 2% of fetal bovine serum, 2% of heat-inactivated horse serum, 2% of B27, 2 mM glutamax, and 1% of penicillin-streptomycin
Acknowledgments
Primary cultures of mouse spinal dorsal horn neuron protocol was funded by the National Natural Science Foundation of China (NSFC 31371121, 81400915, and 31671091). This protocol was developed and used in article published by Jiang et al., 2016.
References
- Hu, H. J., Glauner, K. S. and Gereau, R. W. t. (2003). ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol 90(3): 1671-1679.
- Hugel, S. and Schlichter, R. (2000). Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci 20(6): 2121-2130.
- Jiang, B. C., Cao, D. L., Zhang, X., Zhang, Z. J., He, L. N., Li, C. H., Zhang, W. W., Wu, X. B., Berta, T., Ji, R. R. and Gao, Y. J. (2016). CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest 126(2): 745-761.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cao, D., Jing, P., Jiang, B. and Gao, Y. (2017). Primary Culture of Mouse Neurons from the Spinal Cord Dorsal Horn. Bio-protocol 7(1): e2098. DOI: 10.21769/BioProtoc.2098.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










