- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Ex vivo Culture of Adult Mouse Antral Glands
Published: Vol 7, Iss 1, Jan 5, 2017 DOI: 10.21769/BioProtoc.2088 Views: 9378
Reviewed by: Rakesh BamHui ZhuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1255 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 231 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 144 Views
Abstract
The tri-dimensional culture, initially described by Sato et al. (2009) in order to isolate and characterize epithelial stem cells of the adult small intestine, has been subsequently adapted to many different organs. One of the first examples was the isolation and culture of antral stem cells by Barker et al. (2010), who efficiently generated organoids that recapitulate the mature pyloric epithelium in vitro. This ex vivo approach is suitable and promising to study gastric function in homeostasis as well as in disease. We have adapted Barker’s protocol to compare homeostatic and regenerating tissues and here, we meticulously describe, step by step, the isolation and culture of antral glands as well as the isolation of single cells from antral glands that might be useful for culture after cell sorting as an example (Fernandez Vallone et al., 2016).
Keywords: ex vivoBackground
Mouse adult stem cells from the glandular stomach can be grown ex vivo in a 3D matrigel as ‘mini-glands’ for indefinite periods of time (Barker et al., 2010). As compared to stem cells from the mouse adult small intestine growing in presence of EGF, Noggin and R-spondin 1, gastric stem cells need to be further supplemented with Fgf10, Gastrin, Wnt3a and a higher concentration of R-spondin 1 (referred to as ENRFGW) to get productive cultures. Till recently, whether, and if so how, adult regenerating antral glands grow in the ex vivo culture system following stem cell ablation, remained unknown. Using the present protocol, it was demonstrated that homeostatic and regenerating antral glands do not grow similarly upon seeding and exhibit different growth culture requirements.
Materials and Reagents
- Disposable scalpels (Swan Morton, catalog number: 0510 )
- Petri dishes 92 x 16 mm with cams (SARSTEDT, catalog number: 82.1473 )
- Tubes 50 ml, 30 x 115 mm, PP (Corning, Falcon®, catalog number: 352070 )
- 20 ml eccentric tip syringe (BD, catalog number: 300613 )
- Needle 21 G x 1 ½ (BD, catalog number: 305167 )
- Tips refill (VWR, catalog numbers: 89079-464 ; 89079-470 ; 89079-478 )
- 70 µm nylon filters (Corning, Falcon®, catalog number: 352350 )
- 40 µm nylon filters (Corning, Falcon®, catalog number: 352340 )
- P6 well plate (VWR, catalog number: 734-2323 )
- P12 well plate (VWR, catalog number: 734-2324 )
- Microcentrifuge tubes, 1.5 ml (VWR, catalog number: 212-0198 )
- Tubes 10 ml, 100 x 16 mm, PP (SARSTEDT, catalog number: 62.9924.284 )
- Cryotubes 1 ml (Greiner Bio One, catalog number: 123263 )
- Syringe filter 0.2 µm (VWR, catalog number: 28145-477 )
- Serological pipets 5 ml, 10 ml and 25 ml (Corning, Falcon®, catalog numbers: 357543 ; 357551 ; 357535 )
- Mice (RjOrl:SWISS and C57BL/6JRj backgrounds-6 to 8 weeks old-males and females)
- Dulbecco’s phosphate-buffered saline (DPBS), CaCl2 free, MgCl2 free (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-094 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10270 )
- Stem Pro Accutase cell dissociation reagent (Thermo Fisher Scientific, GibcoTM, catalog number: A1110501 )
- Matrigel® basement membrane matrix (Corning, catalog number: 354234 )
- Liquid nitrogen (supplied from Air liquide)
- 500 mM EDTA (pH 8.0) (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15575-038 )
- Albumin from bovine serum (BSA) (Sigma-Aldrich, catalog number: A3294 )
- Advanced DMEM/F12 (Thermo Fisher Scientific, GibcoTM, catalog number: 12634-010 )
- Gentamycin 50 mg/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15750-037 )
- Penicillin-streptomycin cocktail 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122 )
- Amphotericin B 250 µg/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15290-026 )
- L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030-081 )
- N-2 supplement 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 17502-048 )
- B-27 w/o vit. A 50x (Thermo Fisher Scientific, GibcoTM, catalog number: 12587-010 )
- 1 M HEPES (Thermo Fisher Scientific, GibcoTM, catalog number: 15630-056 )
- N-acetyl cysteine (Sigma-Aldrich, catalog number: A7250 )
- Growth factors:
Recombinant murine EGF (Peprotech, catalog number: 315-09 )
Recombinant murine Noggin (Peprotech, catalog number: 250-38 )
Recombinant murine CHO-derived R-spondin 1 (R&D Systems, catalog number: 7150-RS/CF )
Recombinant murine Fgf10 (R&D Systems, catalog number: 6224-FG )
Recombinant murine Wnt3a (R&D Systems, catalog number: 1324-WN/CF )
Gastrin I (Sigma-Aldrich, catalog number: SCP0152 )
Rho kinase inhibitor Y27632 (Sigma-Aldrich, catalog number: Y0503 ) - DMSO (Sigma-Aldrich, catalog number: D8418 )
- Propanol-2 (VWR, catalog number: 1.09634.9900 )
- Ethanol 95-97% (VWR, TechniSolv®, catalog number: 84857.360 )
- 70% ethanol (see Recipes)
- DPBS-EDTA (10 mM) (see Recipes)
- DPBS-BSA 2%-EDTA (2 mM) (see Recipes)
- Basal crypt medium (BCM) (see Recipes)
- ENRGWF medium for initial seeding (see Recipes)
- ENRGWF medium for maintenance (see Recipes)
- Freezing medium (see Recipes)
- De-freezing medium (see Recipes)
Equipment
- Binocular (Motic, model: SMZ-168 )
- Cold light source (SCHOTT, model: KL1500 LCD )
- Scissors: straight sharp tip (Fine Science Tool, catalog numbers: 14090-09 and 14084-08 )
- Angled serrated tip forceps (Fine Science Tool, catalog number: 11080-02 )
- Standard (fine) tip forceps (Fine Science Tool, catalog number: 11251-20 )
- Micro-dissecting scissors (Fine Science Tool, catalog number: 15018-10 )
- Refrigerated centrifuge (Beckman Coulter, model: Allegra X-15R )
- MaxQTM 4000 shaker with adaptable temperature (Thermo Fisher Scientific, Thermo ScientificTM, model: MaxQTM 4000)
- Biological safety cabinet (Esco Micro Pte, model: Class II Type A2 )
- Pipettors with Tip Ejector 20-200 µl and 100-1,000 µl (VWR, catalog numbers: 89079-970 and 89079-974 )
- Cell culture incubator (37 °C, 5% CO2) (BINDER, model: C150 )
- Inverted bright field microscope (Motic, model: AE31 )
- Nalgene Cryo ‘Mr Frosty’ freezing container (Thermo Fisher Scientific, Thermo ScientificTM, model: 5100-0050 )
- Ultra-low temperature upright freezer (Thermo Fisher Scientific, model: Thermo scientific Queue Basic)
- Cryostorage system K Series (Taylor-Wharton, model: 24K )
Procedure
Notes:
- General considerations regarding mice: Animals should be housed in a temperature (21 ± 1 °C) and humidity (55 ± 10%) - controlled room with a 12 h light/12 h dark cycle.
- Unless specified, steps are carried out at room temperature (RT).
- Dissection procedures and preparation of samples
- Isolation of antral glands for culture (Figure 1)
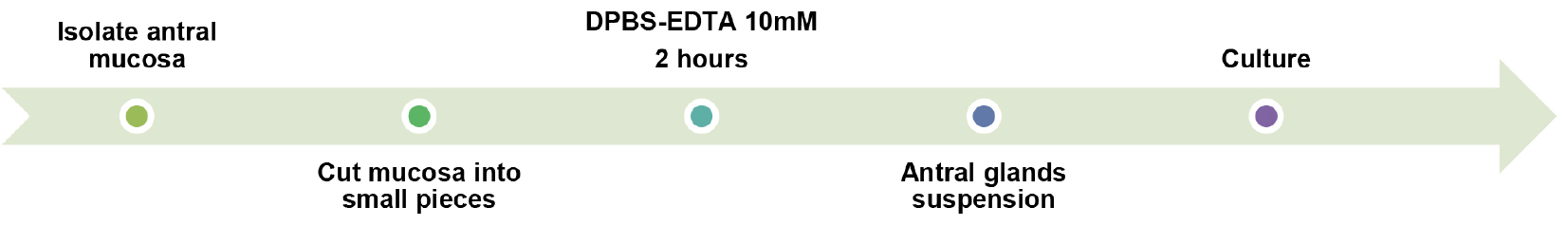
Figure 1. Overall procedure schematic of step A1 - General preparations before starting the dissection
Sterilize working area and dissection tools with 70% ethanol. It is not necessary to work under laminar flow, meanwhile all the steps are carried out carefully and in a clean disinfected area. It is recommended to maintain dissection tools in a glass with 70% ethanol during intermediate steps of the dissection protocol to minimize contamination.
Equipment: binocular and cold light source (Figure 2a).
Dissection tools: scissors, curved forceps, standard tip forceps, scalpel, micro-dissection scissors, dissection bed (Figure 2b).
Box with ice, plastic Petri dishes, ice cold DPBS and 50 ml tubes according to the number of mice that will be processed.
Figure 2. Examples of equipment (a) and dissection tools (b). a. Binocular and cold light source; b. Dissection tools: micro-dissection scissors, scalpel, straight thin tip forceps, curved serrated forceps and different size scissors. - Euthanize the mouse according to the local institutional guidelines.
- Lay the mouse on its back and fix it to the dissection bed. Spray the abdominal area with 70% ethanol in order to sterilize the area and minimize further contamination.
- Grasp the skin with an angled forceps and cut transversally through the skin and peritoneum at the level of the lower abdomen. Perform another 2 cuts on each side in order to be able to lift up the skin and visualize internal organs until the diaphragm (Figure 3a).
- Dissect out with curved forceps and scissors the whole stomach and clean it from rests of other organs (pancreas, duodenum, esophagus, fascias, etc.) and connective tissue (Figures 3b-3d).
- Place the stomach on a Petri dish filled with ice-cold DPBS and cut off the squamous area (Figure 3e).
- Wash the inside stomach with DPBS to remove the rests of partially digested food. This cleaning process may be helped by flushing cold DPBS into the stomach through the open squamous area towards the pylorus and vice-versa with a syringe plus needle.
- With the stomach cleaned and flattened, make a cut with the scalpel to separate corpus from antral area. There is a clear delineation and separation between both areas. Keep antral area (Figures 3e-3f).
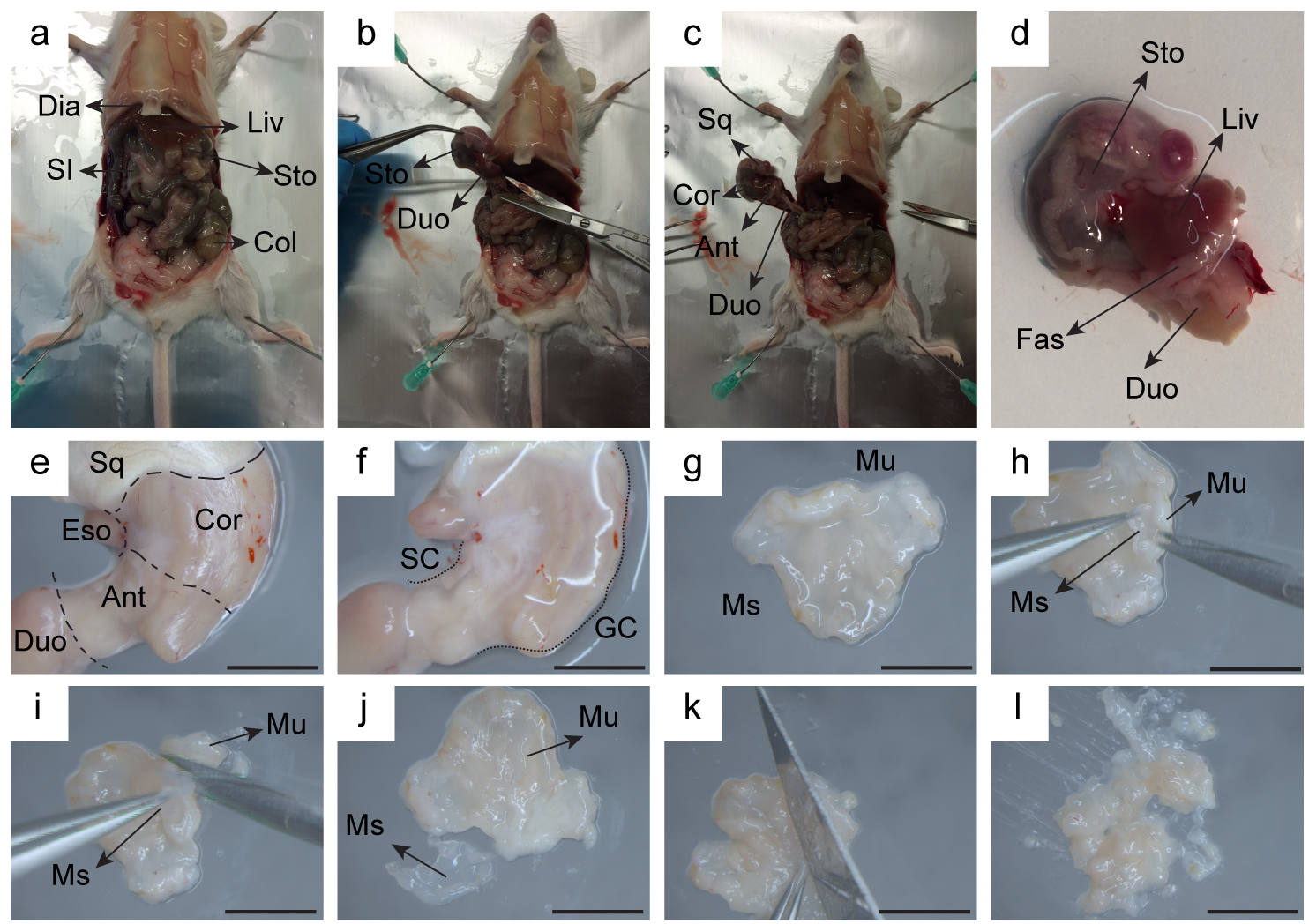
Figure 3. Representative pictures of stomach dissection. Consecutive steps are detailed (a-l). Ant: antral area, Cor: corpus area, Col: colon, Duo: duodenum, Dia: diaphragm, Eso: esophagus, Fas: fascias, GC: greater curvature, Liv: liver, Mu: mucosa, Ms: muscle, SC: small curvature, SI: small intestine, Sq: squamous area, Sto: stomach. Scale bars = 0.5 cm. - Open the antral stomach along the small curvature and wash once more with ice-cold DPBS.
- Place the open antral tissue on a dry Petri dish with the muscle layer facing up and the mucosa down to the Petri (Figure 3g).
- Separate serosal muscle from antral mucosa under the binocular using micro-dissecting scissors and fine forceps. During separation of the serosal muscle, the mucosa becomes more fragile and relaxed (Figures 3h-3j).
- When whole muscle tissue is separated, cut the mucosa into small pieces (< 5 mm2) with the scalpel (Figures 3k-3l).
- Transfer the tissue to a 50 ml tube containing 10 ml sterile ice cold DPBS and place it on ice.
- Continue the dissection of the rest of the mice always keeping the tissue already processed on ice.
- Centrifuge the tubes at 230 x g for 5 min, 4 °C.
- Re-suspend the pellet of each sample in 10 ml of sterile 10 mM DPBS-EDTA.
- Place the tubes laid down on ice and incubate them for 2 h with 75 rpm agitation. Incubation with DPBS-EDTA detaches the epithelial layer from the mesenchymal one.
- Centrifuge the tubes at 230 x g for 5 min, 4 °C.
- From this step onward, the protocol should be carried out under a tissue culture hood.
- Remove supernatant by aspiration and re-suspend each pellet in 10 ml of sterile DPBS.
- Prepare FBS-coated pipette by pipetting up and down once FBS from 50 ml tube containing complete FBS. Empty the pipette so that it is ready to use.
- Pipet 40 times up and down samples using FBS-coated pipette of 10 ml to disrupt the tissue and further separate the antral glands from the basal layer in contact with mesenchyme. Process each tube at a time maintaining the rest on ice.
- Centrifuge the tubes at 300 x g for 5 min, 4 °C.
- Remove supernatant by aspiration and re-suspend each pellet in 5 ml of sterile DPBS.
- Pipet 40 times up and down samples using FBS-coated pipette of 5 ml to disrupt the tissue and further separate the antral glands from the basal layer in contact with mesenchyme. Process each tube at a time maintaining the rest on ice.
- Centrifuge the tubes at 300 x g for 5 min, 4 °C.
- Pass each suspension through a 70 µm filter into a new tube.
- Centrifuge the tubes at 300 x g for 5 min, 4 °C.
- Remove supernatant. Pellet is ready to be seeded in culture (see step B).
- Isolation of antral glands for culture (Figure 1)
Note: These steps allow the isolation of adult antral glands that can be used to give rise to gastric organoids in culture (Figure 4). However, single cell isolation from antral glands might be the best option to perform culture after FACS for example. In this case, the user of this protocol should follow the modification described in step A2 (see below).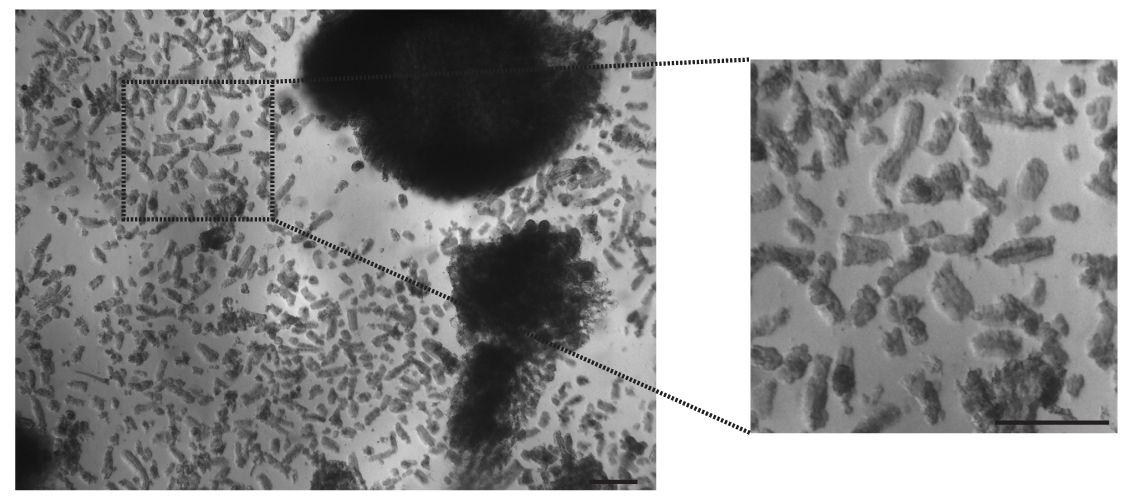
Figure 4. Adult glands isolation. Example of mouse antral glands suspension before plating or single cell dissociation. Scale bars = 100 µm.
- Single cell isolation from antral glands (Figure 5)
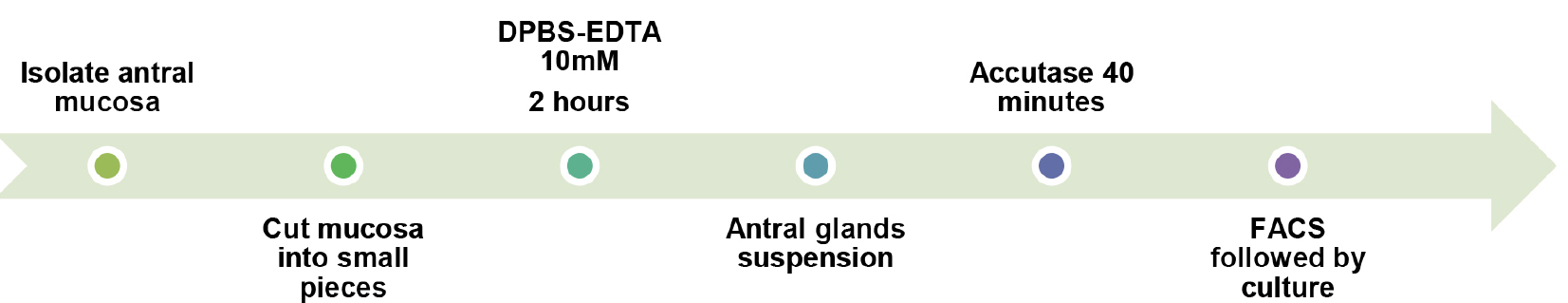
Figure 5. Overall procedure schematic of step A2 - Re-suspend the pellets from step A1z.iii in 4 ml Stem Pro Accutase cell dissociation reagent.
- Transfer each suspension to a P6 well plate and incubate it at 37 °C with 75 rpm agitation for 40 min. It is recommended to check the dissociation every 10 min under the inverted microscope and to help this process with mechanical up and down pipetting (micropipette P1000).
- When single cell suspension is reached, pass it through a 40 µm filter into a new tube.
- Centrifuge the tubes at 300 x g for 5 min, 4 °C.
- Remove supernatant and re-suspend the pellets in 2 ml ice cold 2 mM DPBS-BSA 2%-EDTA solution to wash.
- Repeat the wash twice.
- Finally re-suspend the single cell preparation in 1 ml 2 mM DPBS-BSA 2%-EDTA. Maintain the cell suspension on ice and proceed with staining steps or direct sorting in case of fluorescent protein expression. It is recommended to pass the suspension through a 40 µm filter once again before sorting.
- Single cells of interest can finally be centrifuged at 300 x g for 5 min, 4 °C. Remove supernatant and if desired, proceed with sample seeding for ex vivo culture.
- Ex vivo culture – 3D
- General preparations before starting
Defreeze Matrigel aliquots on ice and keep them always on ice, small changes in the temperature might accelerate undesired polymerization. - Choose the surface of plating and amount of Matrigel for each sample according to the size of the pellet obtained in step A. For individual samples of antral glands suspension, it is recommended to use 1 well of a 6 well plate and 240 µl Matrigel per pellet, however for sorted samples, 1 well of a 12 well plate and 100 µl of Matrigel per pellet might be preferred. All these estimations may change according to the user’s plans and needs.
- Re-suspend the pellet in the tube with the adequate amount of Matrigel and homogenize the suspension on ice.
- Transfer the mix sample/Matrigel suspension to the plate as a drop. Stretch the drop from the center until the bottom of the well is covered without touching the walls (use a tip for this purpose) (Figure 6a).
- Place the plate in an incubator at 37 °C for 10 min until the mix polymerizes.
- Distribute the ENRGWF medium for initial seeding (see Recipes section): 700 µl per well (12 well plate) and 1.4 ml per well (6 well plate).
- Place the plate in the incubator.
- Medium should be fully changed every other day with ENRGWF medium for maintenance (see Recipes section).
- General preparations before starting
Note: All steps, including the decision of plating according to density of sample should be followed by observation under inverted bright field microscope.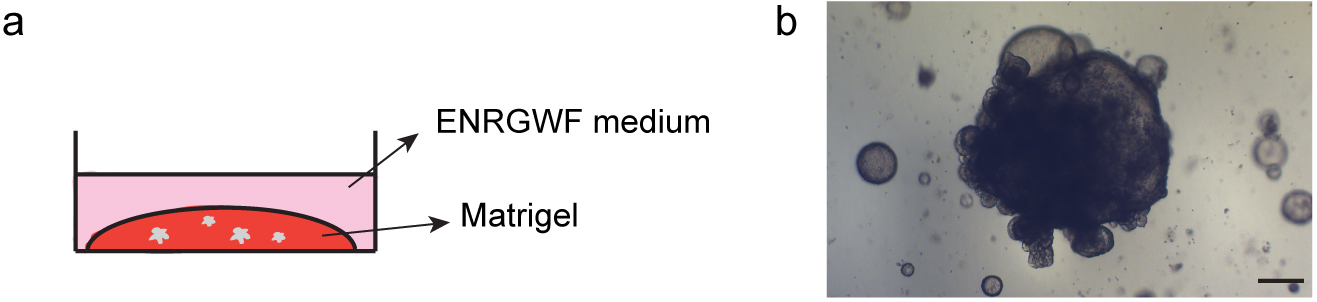

Figure 6. Antral organoids at day 5 of the initial seeding: scheme showing side view of the 3D culture (a) and representative picture of culture at day 5 (b). Scale bars = 100 µm.
- Maintenance of organoids
- General preparations before starting
Defreeze Matrigel aliquots on ice and keep them always on ice, small changes in the temperature might accelerate undesired polymerization.
After 7-10 days in culture (depending on the rate of growth) organoids should be replated (Figure 6b). The replating can be done by ‘picking’ selected elements (step C2) or by harvesting a complete part of the well (step C3). See below details for each case. - Replating by element selection
- Select elements to be picked up under the inverted microscope.
- Prepare one microcentrifuge tube per sample with 1 ml DPBS, pick up the elements from the well with a (P200) and transfer them to the tube with DPBS. Repeat the process for all the desired elements.
- Centrifuge the tube at 300 x g for 5 min.
- Remove supernatant and re-suspend the elements in 0.3 ml DPBS.
- Disrupt the elements mechanically by pipetting up and down with a micropipette (P200) until visual disappearance of big pieces.
- Add 1 ml DPBS to further wash the suspension.
- Centrifuge the tube at 300 x g for 5 min.
- Remove supernatant and re-suspend the pellet in the adequate volume of Matrigel as described in step B.
- Repeat steps B3-B7.
- Replating without selection
- Aspirate the medium from the well.
- Add 1 ml DPBS to the well (P6 or P12 well plate) and detach the whole Matrigel with the elements embedded from the well by pipetting up and down with a micropipette (P1000) until homogeneous suspension is reached.
- Take ¼ from the suspension and transfer it to a 10 ml tube. It is not recommended to replate the whole well into the same size well as it might be too much material and debris, ending with a bad quality of replating. Depending on user’s needs, the rest of the material can be frozen, replated if culture needs to be amplified or discarded.
- Wash with 1 ml DPBS and centrifuge it at 300 x g for 5 min.
- Remove supernatant and re-suspend the elements in 0.3 ml DPBS.
- Disrupt the elements mechanically by pipetting up and down with a micropipette (P200) until visual disappearance of big pieces.
- Add 1 ml DPBS to further wash the suspension
- Centrifuge the tube at 300 x g for 5 min.
- Remove supernatant and re-suspend the pellet in the adequate volume of Matrigel as described in step B.
- Repeat steps B3-B7.
- General preparations before starting
- Organoids cryopreservation
- Freezing protocol
- Aspirate the medium from the well.
- Add 1 ml DPBS to the well (P6 or P12 well plate) and harvest the whole Matrigel with the embedded elements by pipetting up and down with a micropipette (P1000) until homogeneous suspension is reached. If some material is left in the well, harvest it with extra 1 ml DPBS to the same collection tube.
- Centrifuge the tube at 300 x g for 5 min.
- Remove supernatant and wash the pellet in 2 ml DPBS.
- Centrifuge the tube at 300 x g for 5 min.
- Remove supernatant and re-suspend the pellet in 1 ml of freezing medium (see Recipes section).
- Transfer the suspension to a labeled cryotube and place it in the Cryo freezing container (filled with isopropanol at RT). Put the container in a -80 °C freezer.
- After 48 h, cryotubes can be stored in liquid nitrogen.
- De-freezing protoco
- Prepare a tube with 2 ml of de-freezing medium (see Recipes section) and warm it at 37 °C.
- Take out from liquid nitrogen the selected cryotube and de-freeze the sample by pipetting up and down with pre-warmed de-freezing medium.
- Transfer all the organoid suspension to the pre-warmed tube and centrifuge it at 300 x g for 5 min.
- Remove supernatant and wash the pellet in 2 ml of BCM medium twice and centrifuge it at 300 x g for 5 min.
- Remove supernatant and re-suspend the elements in 0.3 ml DPBS.
- Disrupt the elements mechanically by pipetting up and down 20 times with a micropipette (P200).
- Add 1 ml DPBS to further wash the suspension.
- Centrifuge the tube at 300 x g for 5 min.
- Remove supernatant and re-suspend the pellet in the adequate volume of Matrigel as described in step B.
Data analysis
Details of replicates are provided in the original research paper published in free access (Fernandez Vallone et al., 2016).
Notes
- In order to optimize reproducibility, it is suggested to use age-matched animals.
- In order to improve cell viability after cell sorting, single sorted cells are collected in the BCM medium containing 10 µM Y27632.
Recipes
- 70% ethanol
70% ethanol (v/v) in dI water - DPBS-EDTA (10 mM)
1 ml 500 mM EDTA in DPBS
Final volume: 50 ml - DPBS-BSA 2%-EDTA (2 mM)
1 g BSA
0.1 ml 500 mM EDTA in DPBS
Final volume: 50 ml
Pass the solution through a 0.22 µm filter - Basal crypt medium (BCM)
500 ml Advanced DMEM/F12 supplemented with:
0.4 ml gentamycin
5 ml penicillin-streptomycin cocktail stock
5 ml amphotericin B
5 ml L-glutamine (final concentration: 2 mM) - ENRGWF medium for initial seeding
BCM supplemented with:
0.5 ml N-2
1 ml B-27 w/o vit. A
0.5 ml 10 mM HEPES
0.1 ml 1 mM N-acetyl cysteine
Growth factors at a final concentration of: 50 ng/ml for EGF, 100 ng/ml for Noggin, 200 ng/ml for CHO-derived R-spondin 1, 100 ng/ml Fgf10, 100 ng/ml Wnt3a, 10 nM Gastrin and 10 µM Rho kinase inhibitor (Y-27632)
Final volume: 50 ml - ENRGWF medium for maintenance
BCM supplemented with:
0.5 ml N-2
1 ml B-27 w/o vit. A
0.5 ml 10 mM HEPES
0.1 ml 1 mM N-acetyl cysteine
Growth factors at a final concentration of: 50 ng/ml for EGF, 100 ng/ml for Noggin, 200 ng/ml for CHO-derived R-spondin 1, 100 ng/ml Fgf10, 100 ng/ml Wnt3a and 10 nM Gastrin
Final volume: 50 ml - Freezing medium
BCM
1 ml FBS
1 ml DMSO
Final volume: 10 ml - De-freezing medium
BCM
1 ml FBS
Final volume: 10 ml
Acknowledgments
This work was supported by the Interuniversity Attraction Poles Programme-Belgian State-Belgian Science Policy (6/14), the Fonds de la Recherche Scientifique Médicale of Belgium, the Walloon Region (program CIBLES) and the non-for-profit Association Recherche Biomédicale et Diagnostic. This protocol was adapted from the initial report of Barker et al. (2010) for ex vivo culture conditions.
References
- Barker, N., Huch, M., Kujala, P., van de Wetering, M., Snippert, H. J., van Es, J. H., Sato, T., Stange, D. E., Begthel, H., van den Born, M., Danenberg, E., van den Brink, S., Korving, J., Abo, A., Peters, P. J., Wright, N., Poulsom, R. and Clevers, H. (2010). Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6(1): 25-36.
- Fernandez Vallone, V., Leprovots, M., Strollo, S., Vasile, G., Lefort, A., Libert, F., Vassart, G. and Garcia, M. I. (2016). Trop2 marks transient gastric fetal epithelium and adult regenerating cells after epithelial damage. Development 143(9): 1452-1463.
- Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., van Es, J. H., Abo, A., Kujala, P., Peters, P. J. and Clevers, H. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244): 262-265.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Vallone, V. F., Leprovots, M., Vassart, G. and Garcia, M. (2017). Ex vivo Culture of Adult Mouse Antral Glands. Bio-protocol 7(1): e2088. DOI: 10.21769/BioProtoc.2088.
Category
Stem Cell > Adult stem cell > Maintenance and differentiation
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link