- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of THY1+ Undifferentiated Spermatogonia from Mouse Postnatal Testes Using Magnetic-activated Cell Sorting (MACS)
Published: Vol 6, Iss 24, Dec 20, 2016 DOI: 10.21769/BioProtoc.2072 Views: 12110
Reviewed by: Jyotiska ChaudhuriShravani MukherjeeManuel SarmientoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 496 Views
Abstract
In mammals, homeostasis of many tissues rely on a subpopulation of cells, referred to as stem cells, to sustain an appropriate number of undifferentiated and differentiated cells. Spermatogonial stem cells (SSCs) provide the fundamental cellular source for spermatogenesis and are responsible for the lifelong maintenance of the germline pool in testes throughout the reproductive lifespan of males. To gain insight into germline stem cell biology and develop strategies for infertility treatment, several germ cell isolation methods have been reported in order to acquire good quality and quantity of undifferentiated spermatogonia. Among them, magnetic-activated cell sorting (MACS) is an efficient cell isolation method that requires less time and less initial cell numbers to obtain an enriched cell population using an antigen-antibody reaction. Thymus cell antigen 1 (THY1, CD90.2) is recognized as a surface marker of undifferentiated spermatogonia in mouse neonatal and adult testes. Here, we describe a protocol for the isolation of one-week-old THY1+ cells and four-week-old THY1+ cells from mouse testes. The isolation procedure consists of three steps: testis collection and single cell suspension, cell labeling using a biotin-conjugated anti-THY1 antibody and magnetic cell separation. Note, this isolation protocol should be completed within five hours to maximize the quality and the amount of living cells.
Keywords: TestisBackground
Co-existence of active and quiescent stem cells is observed in several adult tissues. Adequate balance between quiescence, self-renewal and differentiation is necessary to sustain an appropriate number of undifferentiated stem cells and to avoid premature stem cell exhaustion for the homeostasis of many tissues (Tseng et al., 2015; Wabik and Jones, 2015; Xin et al., 2016). Infertility has become an increasing problem for human couples and a significant portion of male-related infertility cases results from impaired undifferentiated spermatogonia (Boivin et al., 2007; Matzuk and Lamb, 2008). For this reason, SSCs in spermatogenesis, which is a well-characterized stem cell-dependent process (Oatley and Brinster, 2008), is a valuable model to study regulation of tissue homeostasis. Numerous studies have developed germ cell isolation methods in order to gain insight into the biological functions and regulatory networks of undifferentiated spermatogonia. However, in vivo undifferentiated spermatogonia are heterogeneous in their expression of markers including GFRA1, ID4, PLZF and THY1, and different experimental protocols have influences on the enrichment of subpopulations in specific cellular states (Buageaw et al., 2005; Chan et al., 2014; Costoya et al., 2004; Gassei and Orwig, 2013; Hermann et al., 2015; Kubota et al., 2003; Liao et al., 2014). For instance, a large proportion of PLZF+ and THY1+ undifferentiated spermatogonia is found in quiescent phase of the cell cycle in vivo, whereas PLZF+ and THY1+ undifferentiated spermatogonia cultured in mediums supplemented with serum and growth factors are inclined to the proliferation phase (Costoya et al., 2004; Kanatsu-Shinohara et al., 2011; Kubota et al., 2004a; Liao et al., 2014; Sada et al., 2009).
Here, we provide a step-by-step procedure for the isolation of relative quiescent THY1+ undifferentiated spermatogonia from pre-pubertal mice through a serum-free protocol using magnetic-activated cell sorting. This protocol also contains several important information, including cell numbers needed at each step for suitable enzymatic procedures for living germ cell isolation. This protocol should be a valuable tool to obtain large amount of high quality live undifferentiated spermatogonia with specific subpopulation enrichment through the use of antibodies against SSC surface antigens.
Materials and Reagents
- 100 mm Petri dish (Corning, Falcon®, catalog number: 351029 )
- Polypropylene tube:
2 ml tube (Corning, Axygen®, catalog number: MCT-200-C )
5 ml tube (Corning, Axygen®, catalog number: MCT-500-C ) - Centrifuge tubes:
15 ml tube (Corning, Falcon®, catalog number: 352096 )
50 ml tube (Corning, Falcon®, catalog number: 352070 ) - Cell strainer:
5 ml polystyrene tube with cell strainer snap cap (35 µm nylon mesh) (Corning, Falcon®, catalog number: 352235 )
70 µm nylon mesh (Corning, Falcon®, catalog number: 352350 ) - Columns for cell separation:
MS columns (Miltenyi Biotec, catalog number: 130-042-201 )
LS columns (Miltenyi Biotec, catalog number: 130-042-401 ) - Poly-L-Lysine coated slides (Thermo Fisher Scientific, catalog number: 10143265 )
- ARTTM Barrier Hinged Rack pipette tips
P1000 (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2079-HR )
P200 (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2069-05-HR )
P20 (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2149-05-HR ) - C57BL/6N Mice (BioLASCO, catalog number: C57BL/6N )
- Hank’s balanced salt solution (HBSS), calcium, magnesium, no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 14025092 )
- Hank’s balanced salt solution (HBSS), no calcium, no magnesium, no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 14175095 )
- Type IV collagenase (Thermo Fisher Scientific, GibcoTM, catalog number: 17104019 )
- DNase I (RNase-free) (New England Biolabs, catalog number: M0303 )
- Antibodies:
Biotin rat anti-mouse CD90.2 (Clone 30-H12) (BD, catalog number: 553011 )
Biotin rat IgG2b, κ isotype control (Clone A95-1) (BD, catalog number: 553987 )
Anti-CD90.2Biotin antibody (Lot 5150211128) (Miltenyi Biotec, catalog number: 130-101-908 )
Anti-CD49f antibody (BD, catalog number: 551129 )
Isotype antibody (negative control) (BD, catalog number: 551066 )
Anti-PLZF antibody (Santa Cruz Biotechnology, catalog number: sc-28319 )
Anti-VASA antibody (Abcam, catalog number: ab13840 )
AffiniPure Donkey Anti-Rabbit IgG (H+L) (Alexa Fluor® 488) (Jackson ImmunoResearch, catalog number: 711-545-152 )
AffiniPure Donkey Anti-Mouse IgG (H+L) (Alexa Fluor® 488) (Jackson ImmunoResearch, catalog number: 715-545-151 ) - Anti-biotin microbeads (Miltenyi Biotec, catalog number: 130-090-485 )
- PFA (Sigma-Aldrich, catalog number: 158127 )
- Bovine serum albumin (BSA), IgG-free and protease-free (Jackson ImmunoResearch, catalog number: 001-000-162 )
- Normal donkey serum (Abcam, catalog number: ab7475 )
- EDTA, 0.5 M, pH 8.0 (Thermo Fisher Scientific, AnbionTM, catalog number: AM9260G )
- BSA
- Dulbecco’s phosphate-buffered saline (DPBS), no calcium, no magnesium (Thermo Fisher Scientific, GibcoTM, catalog number: 14190144 )
- Trypsin-EDTA, 0.25%, phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 16000044 )
- PE-Cy5-conjugated antibody
- Tween-20 (Sigma-Aldrich, catalog number: P9416 )
- E-HBSS (see Recipes)
- MACS buffer (see Recipes)
- Collagenase solution (see Recipes)
- Trypsin solution (see Recipes)
- F-MACS buffer (see Recipes)
- Staining buffer (see Recipes)
- FACS buffer (see Recipes)
- PBST (see Recipes)
Equipment
- Sterilized forceps and scissors
- Dissecting microscope (Leica MZ16F Stereomicroscope)
- P1000 pipette
- Centrifuge
- Hemocytometer
- Magnetic separator
OctoMACSTM separator for MS columns (Miltenyi Biotec, catalog number: 130-042-109 )
MidiMACSTM separator for LS columns (Miltenyi Biotec, catalog number: 130-042-302 ) - Beckman Coulter FC500 cytometer (Beckman Coulter, model: FC500 )
- Leica TCS SP5 II confocal microscope (Leica Microsystems, model: Leica TCS SP5 II )
Procedure
- Testis collection and single cell suspension
- Remove testes from mice using sterilized forceps and scissors. Collect the testes in a 100 mm Petri dish containing 10 ml of ice-cold HBSS.
- Remove tunica albuginea from the testes to expose seminiferous tubules using sterilized forceps under a dissecting microscope (Figure 1A, upper panel). Transfer seminiferous tubule clumps to a new 100 mm Petri dish containing 10 ml of ice-cold HBSS. Keep the seminiferous tubules on ice.
- Wash seminiferous tubule clumps using ice-cold HBSS to remove debris and suspension cells.
- In order to estimate the rough volume of seminiferous tubule clumps, we transfer these clumps to a 2 ml polypropylene tube to calculate the volumes. Then, transfer seminiferous tubule clumps to a new Petri dish containing at least 3-fold seminiferous tubule clump volumes of room temperature collagenase solution, supplemented with 1 mg/ml type IV collagenase and 5 U/ml DNase I to remove interstitial Leydig cells, blood cells and peritubular myoid cells, and to digest genomic DNA from dead cells, respectively (see Note 1).
Note: We normally collect 0.2-0.3 ml seminiferous tubule clumps from five one-week-old mice and add 1 ml collagenase solution, and obtain 1-1.5 ml seminiferous tubule clumps from five 4-week-old mice and add 6 ml collagenase solution. - Loosen seminiferous tubules using forceps under a dissecting microscope within 20 min at room temperature (~25 °C) to avoid prolonged enzyme incubation (Figure 1A, lower panel).
The procedures of testis collection, tunica albuginea removal and loosening of seminiferous tubules is demonstrated in Video 1.Video 1. Testis collection, tunica albuginea removal and loosening of seminiferous tubules from 4-week-old mice (steps A1-A5) - Wash and clean the dispersed seminiferous tubules twice with at least 3-fold volumes of room temperature E-HBSS for 20 sec with gentle horizontal shaking at 30 rpm.
- Remove E-HBSS, add 3-fold volumes of collagenase solution and incubate at 37 °C for 20 min.
- Discard supernatant and wash dispersed seminiferous tubules by adding 3-fold volumes of room temperature E-HBSS for 20 sec with gentle horizontal shaking at 30 rpm.
- Repeat this wash process thoroughly five times with room temperature E-HBSS buffer for 20 sec with gentle horizontal shaking at 30 rpm to remove suspension cells.
- Transfer seminiferous tubules to a 2 ml polypropylene tube (or to 5 ml polypropylene tube when executing large number of samples). Add 5-fold volumes of room temperature trypsin solution supplemented with 5 U/ml DNase I. Pipette up and down using a P1000 pipette with cut tip to mechanically mince seminiferous tubules in order to obtain single cell suspension (Figure 1B).
- Incubate the tissues at 37 °C for 3 min.
Note: It is important to execute this reaction (steps A10 and A11) within 10 min, since prolonged trypsin solution incubation may cause a proteolytic cleavage of the cell surface protein (see Note 2). - Add 3-fold volumes of room temperature F-MACS to quench the trypsin reaction (see Note 3).
- Transfer suspension cells to a new 50 ml centrifuge tubes.
- Remove residual tissue and filter the suspension using a 70 µm nylon cell strainer. Wash cell strainer with 2-fold volumes of room temperature MACS buffer after adding cell suspension.
- Centrifuge cell suspension at 300 x g for 10 min at 4 °C.
- Re-suspend cells in ice-cold MACS buffer and count the number of cells with a hemocytometer (see Note 4).
- Remove testes from mice using sterilized forceps and scissors. Collect the testes in a 100 mm Petri dish containing 10 ml of ice-cold HBSS.
- Cell labeling and magnetic separation of one-week-old testicular cells
- Centrifuge again at 300 x g for 10 min at 4 °C and aspirate supernatant. Re-suspend cells in 100 µl ice-cold MACS buffer and add 10 µl biotin-conjugated anti-CD90.2 (THY1) antibody per 107 cells isolated from one-week-old mice. We use approximately 5 µg antibody per 100 µl ice-cold MACS buffer.
- Mix well by gentle pipetting and incubate at 4 °C for 15 min with gentle rotation at 30 rpm (see Note 5).
- Wash by adding equal volume ice-cold MACS buffer to remove the unbound primary antibody.
- Centrifuge cell suspension at 300 x g for 10 min at 4 °C.
- Re-suspend cells and wash again with 2 ml ice-cold MACS buffer per 107 cells and centrifuge at 300 x g for 10 min at 4 °C.
- Aspirate supernatant completely and re-suspend cells in 80 µl MACS buffer and add 20 µl microbeads (Miltenyi Biotec) per 107 cells.
- Mix well by gentle pipetting and incubate for 15 min at 4 °C with gentle rotation at 30 rpm.
- Wash by adding 2 ml MACS buffer per 107 cells and centrifuge at 300 x g for 10 min at 4 °C.
- Aspirate supernatant completely and re-suspend up to 108 cells in 1 ml ice-cold MACS buffer.
- Pass testicular cells through a 35 µm nylon cell strainer to remove cell clumps. Approximate 10% cells are lost compared with the cell number after trypsin treatment (step A16).
- Place MS column on magnetic separator. Prepare MS column (Miltenyi Biotec) for cell separation by rinsing with 500 µl ice-cold MACS buffer.
- Apply cell suspension onto the prepared MS columns (see Note 6) and pipette gently every 30 sec to avoid sedimentation and aggregation of the cells before they pass through the column. This ensures the individual cells will pass through the column fluently and effectively.
- Collect the unbound cells that pass through and wash the column three times with 500 µl ice-cold MACS buffer. These cells can serve as a sample to test the efficiency of removing THY1+ cells from the subpopulation, or as a THY1-negative control group.
- Remove MS column from magnetic separator and place it over a new 15 ml centrifuge tube.
- Add 1 ml ice-cold MACS buffer into MS column in order to elute the THY1+ cells.
- Collect the magnetically labeled THY1+ cells by using the plunger supplied with the column (see Notes 5 and 7; Figures 1E-1M for germ cell purity and live/dead cell ratio).
- Centrifuge again at 300 x g for 10 min at 4 °C and aspirate supernatant. Re-suspend cells in 100 µl ice-cold MACS buffer and add 10 µl biotin-conjugated anti-CD90.2 (THY1) antibody per 107 cells isolated from one-week-old mice. We use approximately 5 µg antibody per 100 µl ice-cold MACS buffer.
- Cell labeling and magnetic separation of 4-week-old testicular cells
- Centrifuge again at 300 x g for 10 min at 4 °C and aspirate supernatant. Re-suspend cells in 1 ml ice-cold MACS buffer and add 20 µl biotin-conjugated anti-CD90.2 (THY1) antibody per 108 cells isolated from 4-week-old mice. There is approximately 10 microgram antibody per 1 ml ice-cold MACS buffer.
- Mix well by gentle pipetting and incubate at 4 °C for 15 min with gentle rotation at 30 rpm (see Note 5).
- Wash by adding equal volume ice-cold MACS buffer and centrifuge cell suspension at 300 x g for 10 min at 4 °C.
- Re-suspend cells and wash again with 2 ml ice-cold MACS buffer per 108 cells and centrifuge at 300 x g for 10 min at 4 °C.
- Aspirate supernatant completely and re-suspend cells in 1 ml ice-cold MACS buffer and add 40 µl microbeads (Miltenyi Biotec) per 108 cells.
- Mix well by gentle pipetting and incubate 15 min at 4 °C with gentle rotation at 30 rpm.
- Wash with 2 ml ice-cold MACS buffer per 108 cells and centrifuge at 300 x g for 10 min at 4 °C.
- Aspirate supernatant completely and re-suspend up to 108 cells in 1 ml ice-cold MACS buffer.
- Pass cells through a 35 µm nylon cell strainer to remove cell clumps.
- Place LS column on magnetic separator. Prepare LS column (Miltenyi Biotec) by rinsing with 2 ml ice-cold MACS buffer.
- Apply cell suspension onto the prepared LS columns (see Note 6) and pipette gently every 30 sec to avoid sedimentation and aggregation of the cells before they pass through the column. This ensures the individual cells will pass through the column fluently and effectively.
- Collect unlabeled cells that pass through and wash the column three times with 2 ml ice-cold MACS buffer. These cells can serve as a sample to test the efficiency of removing THY1+ cells from the subpopulation, or as a THY1-negative control group.
- Remove LS column from the magnetic separator and place it over a new 15 ml centrifuge tube.
- Add 2 ml ice-cold MACS buffer into the LS column in order to elute the THY1+ cells.
- Collect the magnetically labeled THY1+ cells by using the plunger supplied with the column (see Notes 5 and 7; Figures 1E-1M for germ cell purity and live/dead cell ratio)
- Centrifuge again at 300 x g for 10 min at 4 °C and aspirate supernatant. Re-suspend cells in 1 ml ice-cold MACS buffer and add 20 µl biotin-conjugated anti-CD90.2 (THY1) antibody per 108 cells isolated from 4-week-old mice. There is approximately 10 microgram antibody per 1 ml ice-cold MACS buffer.
Data analysis
- All analytical results published (Liao et al., 2014; Tseng et al., 2015) with samples isolated by the exact protocol described here were calculated from three to eight independent experiments.
- Most of the isolated 8 dpp THY1+ cells (~80%) display perinuclear PLZF distribution (Tseng et al., 2015), suggesting this subpopulation is relatively quiescence (Buaas et al., 2004; Costoya et al., 2004).
- More than 85% of total isolated cells from this protocol exhibit PLZF and VASA signals. PLZF is recognized as a marker for undifferentiated spermatogonia and VASA is expressed in all germ cells. The high purity of SSC-enriched primary germ cells (without culture) via this isolation protocol is suitable for genome wide high throughput transcriptome and epigenomic analysis (Liao et al., 2014; Tseng et al., 2015).
- For quality control of immunostaining, samples from the luxoid (ZBTB16lu/lu) mutant mice were used as a negative control to validate the specificity of the PLZF antibody. VASA expressing germ cells and non-VASA expressing somatic cells (mouse embryonic fibroblasts) are used as positive and negative controls to validate the specificity of anti-VASA antibody. The strongest signal intensity from IgG control (secondary antibody only) was used as the cutoff line for background noise.
Representative data
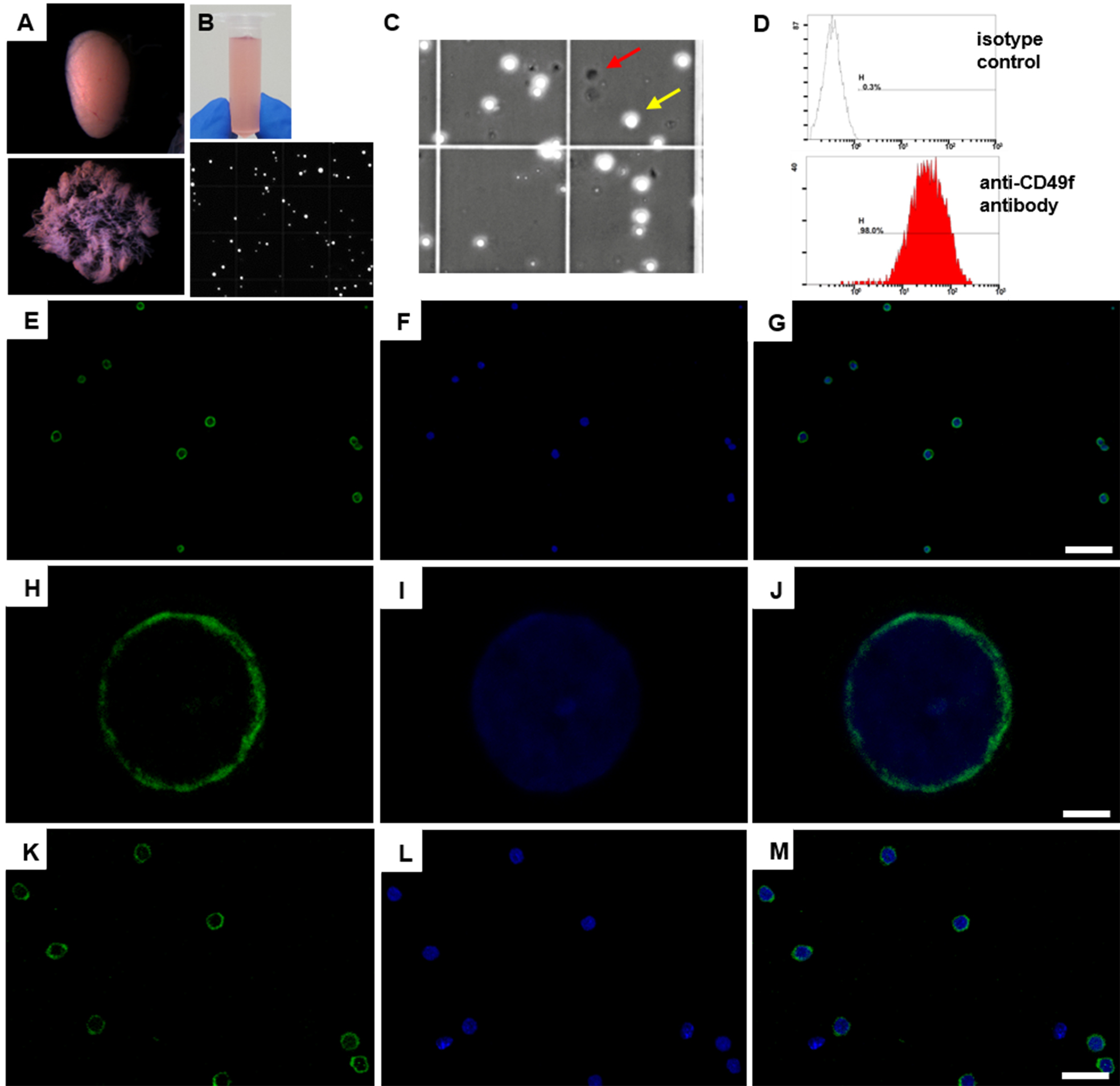
Figure 1. Germ cell enrichment from postnatal mouse testes using magnetic-activated cell sorting. A. Dispersed seminiferous tubules of a testis for an efficient removal of somatic cells outside of seminiferous tubules. Upper panel reveals seminiferous tubule clumps without tunica albuginea. Lower panel indicates dispersed seminiferous tubules. B. A single cell suspension using efficient trypsin solution (pink color, upper panel). Lower panel displays single cell suspension after trypsin treatment under a microscope. C. Each batch of isolated THY1+ cells was treated with trypan blue dye to calculate the percentage of cell death. Unstained viable and round cells (for instance, yellow arrow) are recognized in a phase-contrast microscopy. Red arrow indicates the black color of dead cell. D. Flow cytometry analysis of isolated 8 dpp THY1+ cells labeled with an isotype antibody as negative control (upper panel) and with a PE-Cy5-conjugated anti-CD49f antibody (lower panel) (see Note 8). E-G. Immunostaining of isolated 8 dpp THY1+ cells using a monoclonal anti-PLZF antibody. Green, PLZF; blue, Hoechst 33342. Scale bar = 60 µm. H-J. The PLZF (green) subcellular localization pattern in 80% of isolated 8 dpp THY1+ cells. Green, PLZF; blue, Hoechst 33342. Scale bar = 2.5 µm. K-M. Immunostaining of isolated 4-week-old THY1+ cells using an anti-VASA antibody. VASA predominantly localizes in the cytoplasm. Green, VASA; blue, Hoechst 33342. Scale bar = 25 µm (see Note 9).
Notes
- THY1 is exhibited in various cell types including hematopoietic cells, epithelial cells and fibroblasts. In testes, cells display THY1 expression outside of seminiferous tubules (Kubota et al., 2004b; Rege and Hagood, 2006). Therefore, a thorough removal of somatic THY1+ cells outside of seminiferous tubules is important for the purity of isolated THY1+ undifferentiated spermatogonia (steps A4-A9).
- Trypsin treatment may lead to proteolytic cleavage of surface proteins. To minimize this disadvantage, samples are separated into several groups when there are a large number of testes. F-MACS-treated single cell suspension is kept on ice after being filtered through a 70 µm nylon cell strainer. Add more trypsin solution if cells are clumped or if the reaction solution turns from pink to yellow color, and pipette again to achieve a single cell suspension (Figure 1B).
- Pre-warm the F-MACS buffer to room temperature to minimize cell clumping.
- When five postnatal mice were used for each experiment, testicular cells obtained by this protocol is approximately 1-1.3 x 106 cells per testis at 8 dpp and around 0.7-1 x 107 per testis for 4-week-old mice. The date of birth was defined as 0 dpp.
- Longer antibody incubation at 4 °C for 30 min can increase the recovery rate of THY1+ cells; however, it also increases non-specific binding of anti-CD90.2 antibody to somatic cells (Liao et al., 2014). Based on our experience, the isolation efficiency and purity of undifferentiated spermatogonia varied significantly with different Lot of biotin-conjugated anti-CD90.2 antibodies. For instance, in experienced hands, the ratio of CD49f+ or PLZF+ germ cells is more than 85% of total isolated cells when using Lot 25136 and Lot 80806 of biotin-conjugated anti-CD90.2 antibodies (BD Pharmingen; Figures 1D-1G), whereas the PLZF+ ratio of total isolated cells is between 60% and 85% with Lot 3032695, Lot 5152843 and Lot 6110644 of anti-CD90.2 antibodies (BD Pharmingen) as well as anti-CD90.2Biotin antibody (Miltenyi Biotec, Lot 5150211128). The reduced ratio of germ cells when applying less preferable batches of anti-CD90.2 antibodies may be due to a somatic cell contamination based on the increased VASA-negative cells after immunostaining using an anti-VASA antibody (germ cell marker) (Figures 1K-1M).
In addition, there may be a disadvantage in applying this protocol to isolation of THY1+ undifferentiated spermatogonia from adult mice since there are more THY1+ somatic cells presented in adult testes (Kubota and Brinster, 2008). - In order to perform efficient cell isolation, the typical cell number for MS column should be less than 5 x 107 testicular cells per column and the cell number for a LS column should be less than 5 x 108 testicular cells.
- Dead cells are generally less than 10% of total isolated THY1+ cells determined by trypan blue staining (Figure 1C). An optional centrifugation at 300 x g for 10 min at 4 °C can efficiently remove the dead cells.
- Magnetically isolated THY1+ cells were analyzed through a flow cytometry. A PE-Cy5-conjugated anti-CD49f antibody and an isotype antibody were used for this analysis. THY1+ cells were re-suspended and incubated on ice for 20 min in the dark in staining buffer. After being washed twice with FACS buffer and filtered through a 35 µm nylon cell strainer, PE-Cy5-conjugated cells were analyzed through a Beckman Coulter FC500 Cytometer (Figure 1D).
- For immunocytochemical analysis, isolated THY1+ cells were placed onto Poly-L-Lysine coated slides (Thermo Scientific) and fixed in 4% PFA for 10 min at room temperature. After being washed five times with PBST for 10 min at room temperature, the cells were blocked with 2% BSA and 5% donkey serum for 2 h at room temperature and incubated at 4 °C overnight with the following primary antibody incubation: anti-PLZF antibody or anti-VASA antibody. The cells were washed three times with PBST containing 1% BSA. After incubation with secondary antibodies for 1 h at room temperature, the slides were washed three times in PBST, counterstained with Hoechst 33342 and mounted with mounting medium. The slides were analyzed through a Leica TCS SP5 II confocal microscope (Figures 1E-1M).
Recipes
- E-HBSS
2 mM EDTA in HBSS, no calcium, no magnesium, no phenol red - MACS buffer
2 mM EDTA, 0.5% BSA in DPBS - Collagenase solution
1 mg/ml type IV collagenase and 5 U/ml DNase I in HBSS - Trypsin solution
5 U/ml DNase I in 0.25% trypsin-EDTA, phenol red - Freshly prepared F-MACS buffer
10% FBS in MACS buffer
Note: Keep the F-MACS on ice and pre-warm the F-MACS buffer to room temperature (~25 °C) before use. - Staining buffer
1x DPBS
2 mM EDTA
2% heat-inactivated FBS
PE-Cy5-conjugated antibody (1:1000 dilution) - FACS buffer
1x DPBS
2 mM EDTA - PBST
1x DPBS
0.2% Tween-20
Acknowledgments
The authors would like to express their sincere gratitude to Mr. Pei-Lung Lee, Miss Hung-Chun Tung and Miss Yi-Chun Chen for useful discussions. This work was supported by grants from Ministry of Science and Technology, Taiwan (MOST 103-2321-B-002-099 and MOST 104-2321-B-002-043) and National Taiwan University (NTU-105R4000).
References
- Boivin, J., Bunting, L., Collins, J. A. and Nygren, K. G. (2007). International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22(6): 1506-1512.
- Buaas, F. W., Kirsh, A. L., Sharma, M., McLean, D. J., Morris, J. L., Griswold, M. D., de Rooij, D. G. and Braun, R. E. (2004). Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36(6): 647-652.
- Buageaw, A., Sukhwani, M., Ben-Yehudah, A., Ehmcke, J., Rawe, V. Y., Pholpramool, C., Orwig, K. E. and Schlatt, S. (2005). GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod 73(5): 1011-1016.
- Chan, F., Oatley, M. J., Kaucher, A. V., Yang, Q. E., Bieberich, C. J., Shashikant, C. S. and Oatley, J. M. (2014). Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev 28(12): 1351-1362.
- Costoya, J. A., Hobbs, R. M., Barna, M., Cattoretti, G., Manova, K., Sukhwani, M., Orwig, K. E., Wolgemuth, D. J. and Pandolfi, P. P. (2004). Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 36(6): 653-659.
- Gassei, K. and Orwig, K. E. (2013). SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS One 8(1): e53976.
- Hermann, B. P., Mutoji, K. N., Velte, E. K., Ko, D., Oatley, J. M., Geyer, C. B. and McCarrey, J. R. (2015). Transcriptional and translational heterogeneity among neonatal mouse spermatogonia. Biol Reprod 92(2): 54.
- Kanatsu-Shinohara, M., Inoue, K., Ogonuki, N., Morimoto, H., Ogura, A. and Shinohara, T. (2011). Serum- and feeder-free culture of mouse germline stem cells. Biol Reprod 84(1): 97-105.
- Kubota, H., Avarbock, M. R. and Brinster, R. L. (2003). Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A 100(11): 6487-6492.
- Kubota, H., Avarbock, M. R. and Brinster, R. L. (2004a). Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod 71(3): 722-731.
- Kubota, H., Avarbock, M. R. and Brinster, R. L. (2004b). Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 101(47): 16489-16494.
- Kubota, H. and Brinster, R. L. (2008). Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol 86: 59-84.
- Liao, H. F., Chen, W. S., Chen, Y. H., Kao, T. H., Tseng, Y. T., Lee, C. Y., Chiu, Y. C., Lee, P. L., Lin, Q. J., Ching, Y. H., Hata, K., Cheng, W. T., Tsai, M. H., Sasaki, H., Ho, H. N., Wu, S. C., Huang, Y. H., Yen, P. and Lin, S. P. (2014). DNMT3L promotes quiescence in postnatal spermatogonial progenitor cells. Development 141(12): 2402-2413.
- Matzuk, M. M. and Lamb, D. J. (2008). The biology of infertility: research advances and clinical challenges. Nat Med 14(11): 1197-1213.
- Oatley, J. M. and Brinster, R. L. (2008). Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 24: 263-286.
- Rege, T. A. and Hagood, J. S. (2006). Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 20(8): 1045-1054.
- Sada, A., Suzuki, A., Suzuki, H. and Saga, Y. (2009). The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 325(5946): 1394-1398.
- Tseng, Y. T., Liao, H. F., Yu, C. Y., Mo, C. F. and Lin, S. P. (2015). Epigenetic factors in the regulation of prospermatogonia and spermatogonial stem cells. Reproduction 150(3): R77-91.
- Wabik, A. and Jones, P. H. (2015). Switching roles: the functional plasticity of adult tissue stem cells. EMBO J 34(9): 1164-1179.
- Xin, T., Greco, V. and Myung, P. (2016). Hardwiring stem cell communication through tissue structure. Cell 164(6): 1212-1225.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Liao, H., Kuo, J., Lin, H. and Lin, S. (2016). Isolation of THY1+ Undifferentiated Spermatogonia from Mouse Postnatal Testes Using Magnetic-activated Cell Sorting (MACS). Bio-protocol 6(24): e2072. DOI: 10.21769/BioProtoc.2072.
Category
Cell Biology > Cell isolation and culture > Cell isolation
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link