- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Chondrogenic Hypertrophy Induction of Mesenchymal Stem Cells
Published: Vol 6, Iss 23, Dec 5, 2016 DOI: 10.21769/BioProtoc.2057 Views: 13552
Reviewed by: Vivien Jane Coulson-ThomasAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
Quang Bach Le [...] Deepak Choudhury
Nov 20, 2025 1540 Views

A Protocol to Induce Brown and Beige Adipocyte Differentiation From Murine and Human Adipose-Derived SVF
Rohit Raj Yadav [...] Narendra Verma
Dec 5, 2025 1657 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 232 Views
Abstract
To investigate underlying mechanism of chondrogenic hypertrophy, we need proper in vitro hypertrophic model of mesenchymal stem cells (MSCs). This protocol describes our defined method for induction of in vitro chondrogenic hypertrophy of human umbilical cord blood-derived MSCs (hUCB-MSCs). By adding thyroid hormone (T3; triiodothyronine) and minimum osteogenic-inducing factors to culture medium, we could induce hypertrophy of hUCB-MSCs in vitro. Hypertrophic induction was validated using immunohistochemical analysis, Western blotting and reverse transcriptase polymerase chain reaction.
Keywords: Mesenchymal stem cellBackground
Several studies have shown that expression of hypertrophy-associated genes, including type X collagen, alkaline phosphatase, and parathyroid hormone-related protein receptor (PTHrPR) in chondrogenic differentiation of MSCs. The expression of these genes suggests that chondrogenic differentiation in MSCs inevitably induce chondrogenic hypertrophy stage which is typical of endochondral ossification. In addition, it is known that the activation of the parathyroid hormone-related protein (PTHrP) pathway induces MSC transition to an osteogenic phenotype (Guo et al., 2006). Based on these reports, Mueller et al. suggested that depletion of TGF-β, low concentration of dexamethasone, and addition of triiodothyronine (T3) was important for hypertrophic induction of bone marrow-derived MSCs (Mueller et al., 2008). In Mueller’s protocol, β-glycerophosphate and dexamethasone are necessary to induce higher hypertrophy status. However, their results indicated that treatment of β-glycerophosphate is not essential to induce hypertrophic morphology of chondrocytes. In addition, a recent report showed that dexamethasone has inhibitory effects on hypertrophic induction of MSCs dependent experimental conditions (Shintani et al., 2011). Thus, the use of these agents is not necessarily required to induce hypertrophy. We established a simpler hypertrophy-inducing protocol by withdrawal of β-glycerophosphate and dexamethasone from hypertrophy-inducing culture medium.
Materials and Reagents
- 15 ml sterile conical tubes (Corning, catalog number: 430055 )
- 50 ml sterile conical tubes (Corning, catalog number: 430829 )
- Microslide glass (Thermo Fisher Scientific, Fisher Scientific, catalog number: 22-230-900 )
- 0.22 μm syringe filter (Pall, catalog number: PN4192 )
- Umbilical cord blood-derived mesenchymal stem cells (Neonatal umbilical cord blood was collected from umbilical veins, with informed maternal consent. For UCB collection, a 16-gauge needle was inserted into the umbilical vein, and UCB was allowed to flow by gravity; See Yang et al. [2004] for the protocol of isolation and maintenance of cells)
- Minimum essential medium-alpha (Thermo Fisher Scientific, GibcoTM, catalog number: 12571 )
- Dulbecco’s phosphate buffered saline without calcium & magnesium (Mediatech, catalog number: 21-031-CVR )
- TrypLETM Express enzyme (Thermo Fisher Scientific, GibcoTM, catalog number: 12605 )
- 4% paraformaldehyde solution (Biosesang, catalog number: P2031 )
- Ethanol
- DAKO EnVisionSystem Peroxidase (DAB) Kit (Agilent Technologies, catalog number: K4006 )
- DAKO Protein block, serum-free (Agilent Technologies, catalog number: X0909 )
- Antibody for type II collagen (EMD Millipore, catalog number: MAB8887 , antibody dilution 1:100)
- Antibody for type X collagen (Thermo Fisher Scientific, Invitrogen, catalog number: MA5-14268 , antibody dilution 1:100)
- Antibody for RUNX2 (Abcam, catalog number: ab76956 , antibody dilution 1:1500)
- Antibody for phospho-GSK-3β(Ser9) (Cell Signaling Technology, catalog number: 5558 , antibody dilution 1:1000)
- Antibody for β-Catenin (Cell Signaling Technology, catalog number: 9582 , antibody dilution 1:1000)
- Antibody for phosphor-Smad1(Ser206) (Cell Signaling Technology, catalog number: 5753 , antibody dilution 1:1000)
- Antibody for GAPDH (Abcam, catalog number: ab9485 , antibody dilution 1:4000)
- Tris-HCl
- BSA
- Tween 20 (Sigma-Aldrich, catalog number: P1379 )
- Mayer’s hematoxylin (Agilent Technologies, catalog number: S3309 )
- Shandon Xylene Substitute (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 6764506 )
- Shandon Xylene Substrate Mountant (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 9999122 )
- Fetal bovine serum, certified grade, US origin (Thermo Fisher Scientific, GibcoTM, catalog number: 16000-044 )
- Gentamicin (Thermo Fisher Scientific, GibcoTM, catalog number: 15750 )
- Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 11965 )
- BMP-6 (R&D Systems, catalog number: 507-BP-020/CF )
- TGF-β3 (R&D Systems, catalog number: 243-B3-002/CF )
- ITS+ (Corning, catalog number: 354352 )
- Ascorbic acid (Sigma-Aldrich, catalog number: A8960 )
- Dexamethasone (Sigma-Aldrich, catalog number: D2915 )
- L-proline (Sigma-Aldrich, catalog number: P5607 )
- Sodium pyruvate (Sigma-Aldrich, catalog number: P8574 )
- Triiodothyronine (Sigma-Aldrich, catalog number: T6397 )
- Primers for Runx2
Forward 5’-CGG AGT GGA TGA GGC AAG AG-3’
Reverse 5’-GGC TCA GGT AGG AGG GGT AA-3’ - Primers for GAPDH
Forward 5’- CTT CTT TTG CGT CGC CAG CCG A-3’
Reverse 5’-TGG CCA GGG GTG CTA AGC AGT-3’ - Complete culture media (see Recipes)
- In vitro chondrogenesis-inducing media (see Recipes)
- In vitro hypertrophy-inducing media (see Recipes)
Equipment
- 175T flask (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 159910 )
- Centrifuge with swinging-bucket rotor and adaptors for 15 ml conical tubes
- Humidified cell culture incubator set to 37 °C and 5% CO2
- Hemacytometer (VWR, INCYTO C-ChipTM, catalog number: DHCN015 )
- Cryostat (Leica Biosystems Nussloch, model: CM1850 )
- OCT compound (VWR, Tissue-Tek®, catalog number: 25608-930 )
- Microscope for immunohistochemical staining image analysis (Nikon ECLIPSE 50i with DS-Fi1 digital microscope camera head) (Nikon Instruments, model: ECLIPSE 50i )
Software
- ImageJ program
Procedure
- Plating cells for proliferation
- Seed 5,000 mesenchymal stem cells per cm2 in a 175T flask with complete culture media (50 ml).
- Next day, replace half the medium (25 ml) with fresh complete culture media.
- Exchange the culture medium with fresh minimum essential medium-alpha culture media every 2-3 days until cells are at least 70-80% confluent.
- At 70-80 % confluency, wash the cells with DPBS once and then detach the cells with TrypLETM Express enzyme.
- Seed 5,000 mesenchymal stem cells per cm2 in a 175T flask with complete culture media (50 ml).
- Induction of in vitro chondrogenesis
- For chondrogenesis procedure, wash cells with 30 ml of in vitro chondrogenesis-inducing media (without BMP-6, TGF-β3, and ITS+) twice in a 50 ml tube. For washing, centrifuge suspended cells in the 50 ml tube at 500 x g for 5 min at room temperature.
- Count the cells using a hemacytometer.
- Suspend the cells with in vitro chondrogenesis-inducing media in a 15 ml tube at a final concentration of 2 x 105/400 μl.
- Centrifuge the cells in the 15 ml tube at 500 x g for 5 min at room temperature.
- Be careful not to shake the tube, culture the cell pellet with in vitro chondrogenesis-inducing media in the incubator. Loosen the cap of the tube for gas exchange and keep the tube upright (Figure 1).
- Culture the cell pellet in in vitro chondrogenesis-inducing medium for 2 weeks. Replace the medium with fresh in vitro chondrogenesis-inducing media twice a week (first media change after 3 days and second media change after 4 days).
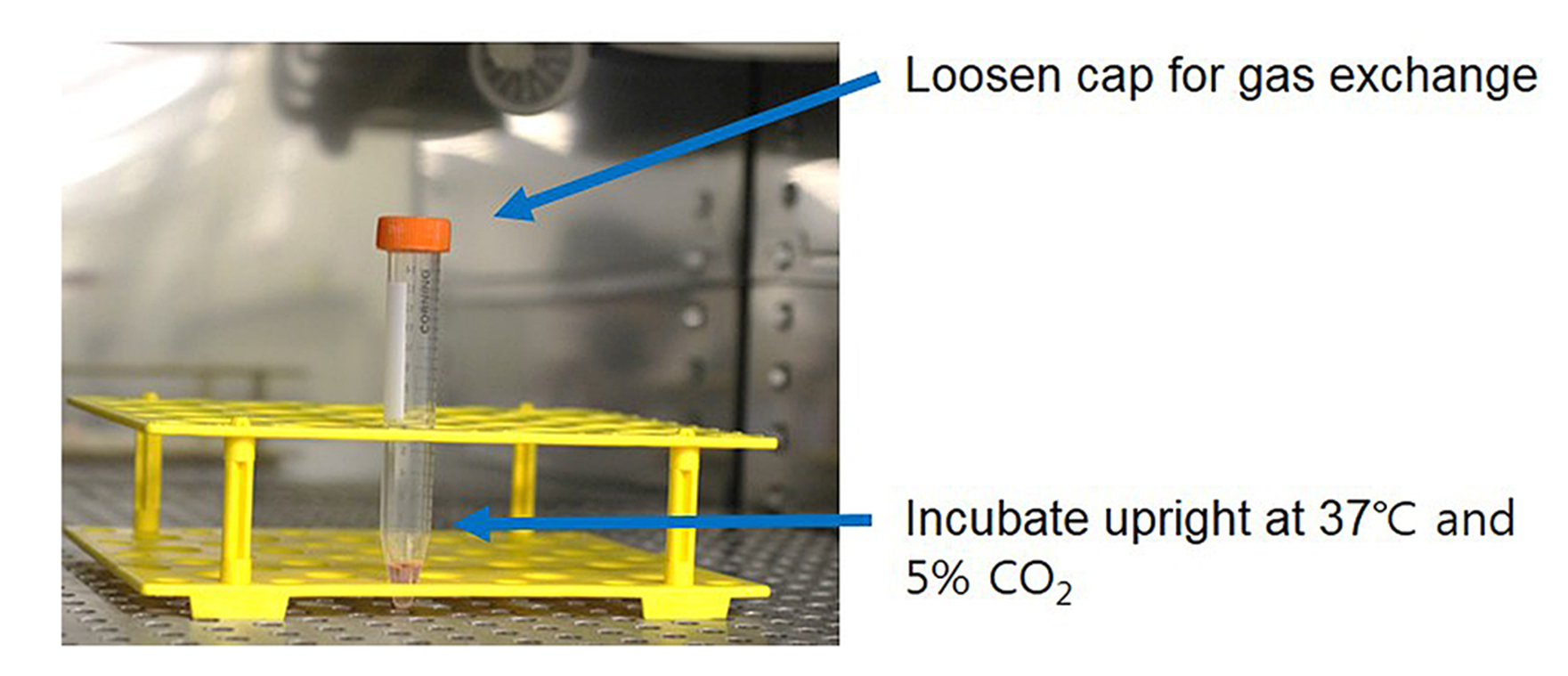
Figure 1. Pellet culture using a 15 ml tube in a CO2 incubator. After centrifugation, the 15 ml tube was carefully moved into a CO2 incubator. During culturing period, the 15 ml tube should be placed upright using a conical tube rack.
- For chondrogenesis procedure, wash cells with 30 ml of in vitro chondrogenesis-inducing media (without BMP-6, TGF-β3, and ITS+) twice in a 50 ml tube. For washing, centrifuge suspended cells in the 50 ml tube at 500 x g for 5 min at room temperature.
- Induction of in vitro hypertrophy
- For hypertrophy induction, wash the cell pellet with in vitro hypertrophy-inducing media twice.
- Add 400 μl of fresh in vitro hypertrophy-inducing media into the 15 ml tube
- Culture the cell pellet with in vitro hypertrophy-inducing media for 2 weeks. Replace the medium with fresh in vitro hypertrophy-inducing media twice a week (first media change after 3 days and second media change after 4 days).
- For hypertrophy induction, wash the cell pellet with in vitro hypertrophy-inducing media twice.
- Immunohistological analysis
- Wash the cell pellet using DPBS and fix using 4% paraformaldehyde solution for 2 h at room temperature.
- Freeze the cell pellet in OCT compound at -20 °C. Cut into 5 μm thick cryostat sections and place on a charged glass microslide.
- Frozen sections are dehydrated for 10 min in ethanol and briefly immersed in distilled water to remove OCT compound.
- To block activity of peroxidase, apply enough Peroxidase Block solution (Component of DAKO DAB Kit) to cover specimen and incubate for 5 min at room temperature. Rinse gently with distilled water.
- To block nonspecific antibody-binding sites, apply enough Protein Block solution to cover specimen and incubate for 30 min at room temperature. Rinse gently with distilled water.
- After washing, the slides are incubated overnight at 4 °C with primary antibody against type X collagen diluted in 0.05 M Tris-HCl with 1% BSA.
- Wash the slides with 500 ml of DPBS (containing 0.1% Tween 20) using a staining jar for 10 min. Repeat one more time.
- Apply enough Labelled Polymer-HRP anti-mouse solution (Component of DAKO DAB Kit) to cover specimen and incubate for 30 min at room temperature.
- Wash the slides with 500 ml of DPBS (containing 0.1% Tween 20) using a staining jar for 10 min. Repeat one more time.
- Wash the slides with 500 ml of DPBS using a staining jar for 10 min. Repeat one more time.
- Apply enough DAB + Chromogen solution (Component of DAKO DAB Kit) to cover specimen.
- Rinse gently with distilled water.
- For counter staining, apply enough Hematoxylin solution to cover specimen and incubate for 1 min.
- Wash the slides with 500 ml of distilled water using a staining jar for 2 min. Repeat two more times.
- Dehydrate the slides consecutively in 70%, 95%, and 100% ethanol for 1 min in each solution.
- Set the slides in 100% ethanol for 2 min and repeat one more time.
- Set the slides in Shandon Xylene Substitute for 2 min and repeat two more times.
- Mount the slides with Shandon Xylene Substrate Mountant.
- Wash the cell pellet using DPBS and fix using 4% paraformaldehyde solution for 2 h at room temperature.
Data analysis
In in vitro chondrogenesis-induction of MSCs, chondrogenic differentiation status was easily estimated by pellet size. After 28 days under chondrogenesis- and hypertrophy-inducing conditions, we measured the size of pellet using a mm scale ruler (Figure 2). The chondrogenic-differentiated (left, Figure 2) and hypertrophic-differentiated (right, Figure 2) pellets are > 1 mm, 0.7-0.8 mm in diameter size, respectively. Our results showed that hypertrophic-differentiated pellet had a smaller size than that of chondrogenic-differentiated pellet of MSCs.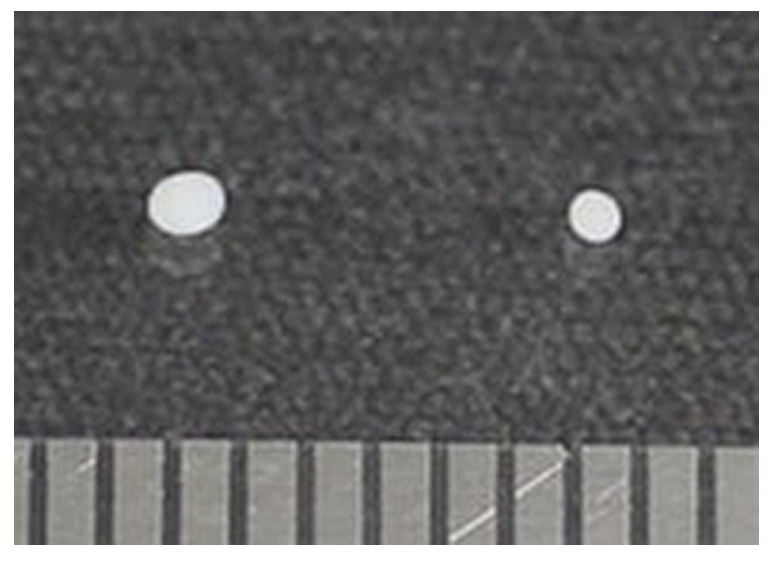
Figure 2. Pellet size comparison. Left pellet of UCB-MSCs was cultured in in vitro chondrogenesis-inducing medium for 4 weeks. Right pellet was cultured in in vitro chondrogenesis-inducing medium for 2 weeks and consecutively cultured in in vitro hypertrophy-inducing medium for 2 weeks. The lower part of picture shows a graduation of mm scale ruler.
To check the hypertrophic induction of pellets under each condition, we detected expression of type X collagen by immunohistochemical staining (Figure 3). The pellets obtained under chondrogenic-inducing conditions showed a weak positive reactivity to type X collagen antibody (Figure 3A). The pellets cultured in hypertrophy-inducing medium showed a dark brown color that indicated high expression levels of type X collagen (Figure 3B). Collectively, we could observe a higher expression of type X collagen in hypertrophic-differentiated pellet.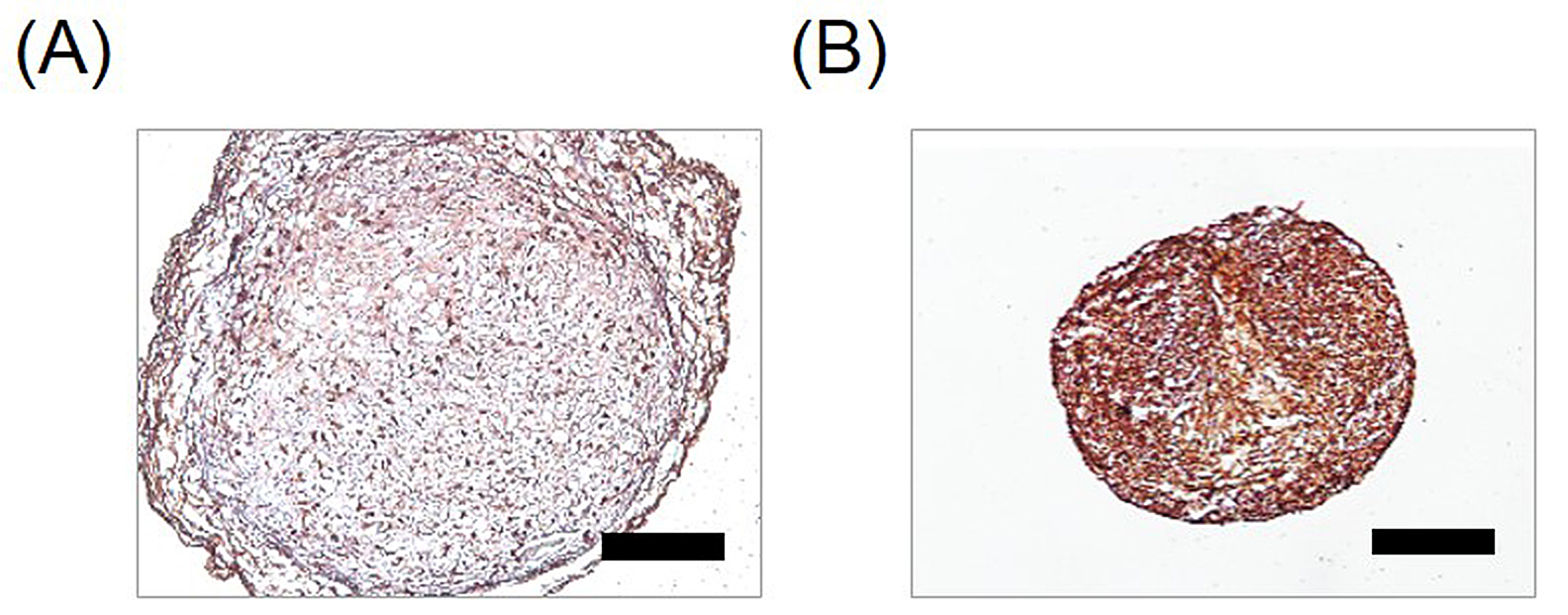
Figure 3. Type X collagen expression in pellets. To validate the induction of hypertrophy in the hUCB-MSCs, the pellets were sectioned and expression of collagen type X was detected by immunohistochemical staining. A. For 4 weeks, pellet was induced into chondrogenic differentiation. B. After 2 weeks chondrogenic differentiation, pellet was induced into hypertrophic differentiation for 2 weeks. Scale bar = 250 μm.
For additional validation of hypertrophic induction, we evaluated the expression of the hypertrophy marker, runt-related transcription factor 2 (Runx2), using Western blotting and RT-PCR (Figure 4). Under hypertrophy-inducing conditions, the protein and mRNA expression level of the osteogenic transcription factor Runx2 were 2.4-fold and 3.1-fold higher than those in pellets under chondrogenic-inducing conditions, respectively. The expression of Runx2 was quantified using ImageJ program.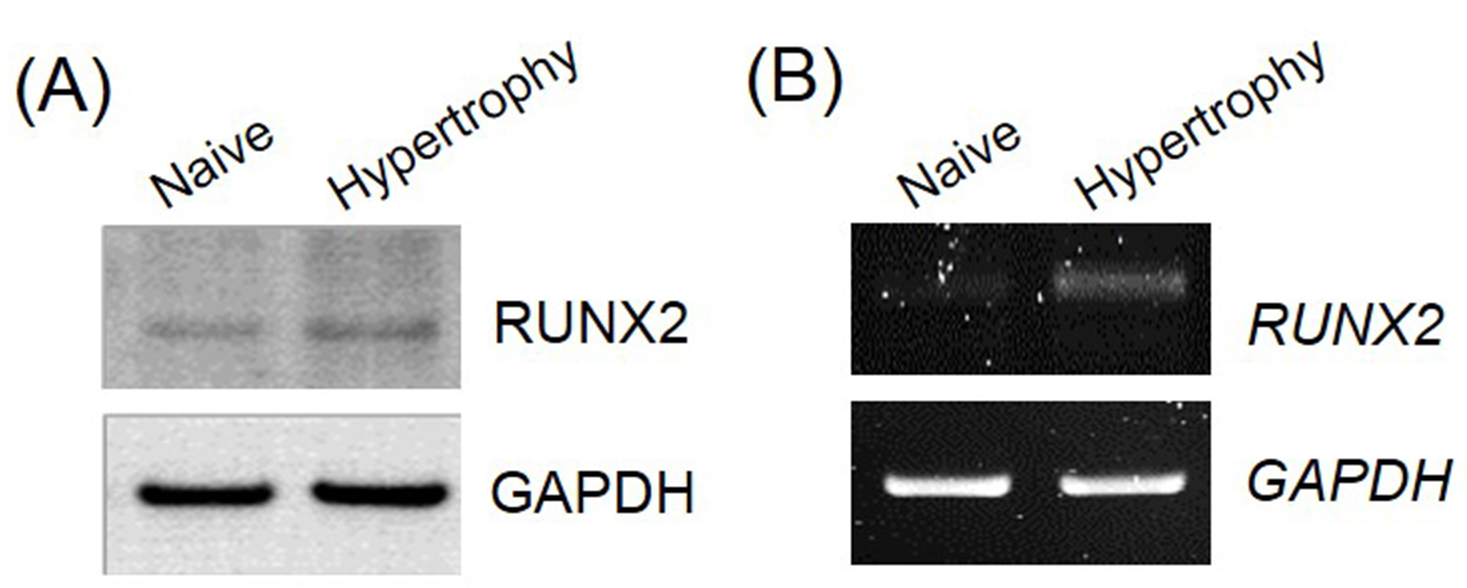
Figure 4. Osteogenic transcription factor expression in pellets. To validate of hypertrophic differentiation in UCB-MSCs, the expression of transcription factor, Runx2, was assessed using Western blotting (A) and reverse transcriptase polymerase chain reaction (B).
To characterize the signal pathway related to hypertrophic induction by our protocol, we evaluated the expression of phosphorylated (p)GSK-3β, β-catenin, and pSMAD1 in the WNT/β-catenin and TGF-β/BMP osteogenic signaling pathways using Western blotting (Figure 5). In hypertrophic-differentiated pellets, pGSK-3β expression was 3.6-fold higher than that of pellet under chondrogenic-inducing conditions. The β-catenin and pSMAD1 expression levels were 2.1-fold and 2.8-fold higher, respectively, than those in chondrogenic-differentiated pellets. The expression of each proteins was quantified using ImageJ program.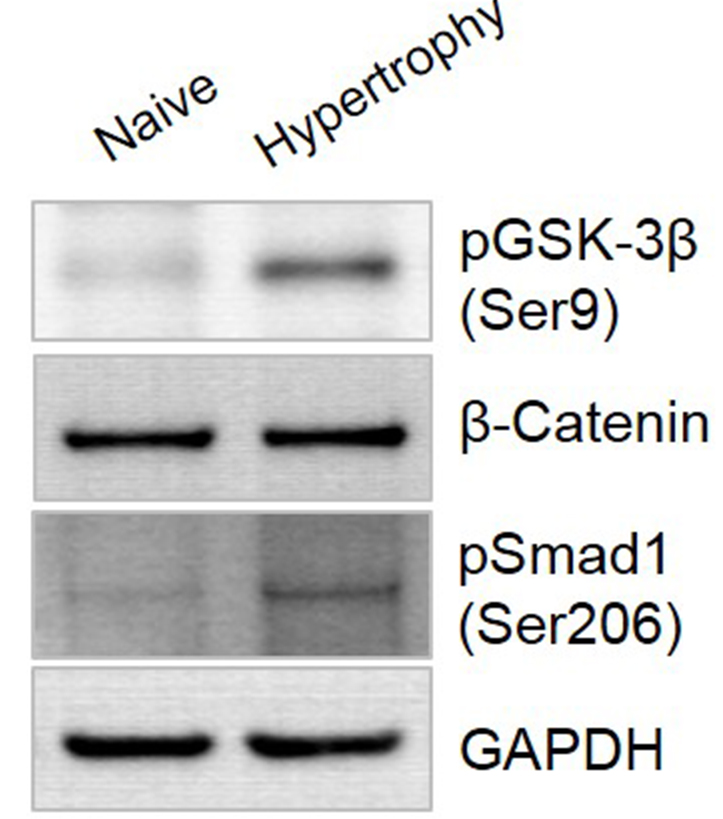
Figure 5. Osteogenic signaling pathway during hypertrophic differentiation of hUCB-MSCs. After hypertrophic cultivation, the pellets were harvested and lysed. The expression of GSK-3β, β-catenin, and SMAD1 was analyzed by Western blotting.
Notes
- If you observe a lower hypertrophy degree of pellets, increase number of washing step in induction of in vitro hypertrophy.
- Use fresh ITS+ supplement within two months of production.
- Avoid repeated freezing and thawing of T3.
Recipes
- Complete culture media (Prepared media can be kept at 4 °C for 2 weeks)
Minimum essential medium-alpha
10% fetal bovine serum (FBS)
50 μg/ml gentamicin - In vitro chondrogenesis-inducing media (Use prepared media immediately for cytokine activity)
500 ml Dulbecco’s modified Eagle medium (DMEM)
500 ng/ml BMP-6
10 ng/ml TGF-β3
1% ITS+
50 μg/ml ascorbic acid
0.6 μg/ml dexamethasone
40 μg/ml L-proline
100 μg/ml sodium pyruvate
50 μg/ml gentamicin - In vitro hypertrophy-inducing media (Use prepared media immediately for cytokine activity)
500 ml Dulbecco’s modified Eagle medium (DMEM)
10% ITS+
50 μg/ml ascorbic acid
40 μg/ml L-proline
1 nM Triiodothyronine
50 μg/ml gentamicin
Acknowledgments
This protocol was modified from our previous work (Jeong et al., 2015) and method of Jason A. Burdick group (Bian et al., 2012) and was supported by the National Research Foundation (2015M3D6A1065098).
References
- Bian, L., Zhai, D. Y., Zhang, E. C., Mauck, R. L. and Burdick, J. A. (2012). Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A 18(7-8): 715-724.
- Guo, J., Chung, U., Yang, D., Karsenty, G., Bringhurst, F. R., Kronenberg H. M. (2006). PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and –independent pathways. Dev Biol 292(1): 116-128.
- Jeong, S. Y., Ha, J., Lee, M., Jin, H. J., Kim, D. H., Choi, S. J., Oh, W., Yang, Y. S., Kim, J. S., Kim, B. G., Chang, J. H., Cho, D. H. and Jeon, H. B. (2015). Autocrine action of thrombospondin-2 determines the chondrogenic differentiation potential and suppresses hypertrophic maturation of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells 33(11): 3291-3303.
- Mueller, M. B. and Tuan, R. S. (2008). Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58(5): 1377-1388.
- Shintani, N. and Hunziker, E. B. (2011). Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater 22: 302-319; discussion 319-320.
- Yang, S. E., Ha, C. W., Jung, M., Jin, H. J., Lee, M., Song, H., Choi, S., Oh, W. and Yang, Y. S. (2004). Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 6(5): 476-486.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jeong, S. Y., Lee, M., Choi, S. J., Oh, W. and Jeon, H. B. (2016). In vitro Chondrogenic Hypertrophy Induction of Mesenchymal Stem Cells. Bio-protocol 6(23): e2057. DOI: 10.21769/BioProtoc.2057.
Category
Stem Cell > Adult stem cell > Mesenchymal stem cell
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link