- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Various Modes of Spinal Cord Injury to Study Regeneration in Adult Zebrafish
Published: Vol 6, Iss 23, Dec 5, 2016 DOI: 10.21769/BioProtoc.2043 Views: 13379
Reviewed by: Oneil G. BhalalaJingli CaoHélène M. Léger

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

zPACT: Tissue Clearing and Immunohistochemistry on Juvenile Zebrafish Brain
Pierre Affaticati [...] Arnim Jenett
Dec 5, 2017 12628 Views

Detection of mRNA by Whole Mount in situ Hybridization and DNA Extraction for Genotyping of Zebrafish Embryos
Rachna Narayanan and Andrew C. Oates
Mar 20, 2019 15743 Views

Quantifying Mechanical Strain–Induced Membrane Damage in Early Neuronal Cells Using an In Vitro Traumatic Brain Injury Model
Gia Kang [...] Andrew R. Harris
Feb 5, 2026 115 Views
Abstract
Spinal cord injury (SCI) in mammals leads to failure of both sensory and motor functions, due to lack of axonal regrowth below the level of injury as well as inability to replace lost neural cells and to stimulate neurogenesis. In contrast, fish and amphibians are capable of regenerating a variety of their organs like limb/fin, jaw, heart and various parts of the central nervous system (CNS). Zebrafish embryo and adult has become a very popular model to study developmental biology, cell biology and regeneration for various reasons. Adult zebrafish, one of the most important vertebrate models to study regeneration, can regenerate many of their body parts like fin, jaw, heart and CNS. In the present article we provide information on how to inflict different injury modalities in adult fish spinal cord. Presently, the significant focus of mammalian SCI is to use crush and contusion injury. To generate an entity comparable to the mammalian mode of injury, we have introduced the crush model in adult zebrafish along with complete transection injury, which is also known to be a valuable model to study axonal regeneration. Here we provide full description of the highly reproducible surgical procedures including some representative results. This protocol has been adapted from our previous publications, viz. Hui et al., 2010 and Hui et al., 2014. Briefly, we have described the two different injury modalities, crush and complete transection, and demonstrated the outcome of inflicting these injuries in the adult zebrafish cord by histological analysis of the tissues.
Keywords: Spinal cordBackground
Any injury to mammalian spinal cord leads to the devastating consequence of paralysis and loss of function. In contrast to mammals, injury response in zebrafish cord is quite different, resulting in repair and regeneration of cord followed by functional recovery. A variety of lesioning protocols have been employed to study spinal cord injury and functional recovery in lower vertebrates (Holtzer 1956; Egar and Singer, 1972; Filoni et al., 1984; Becker et al., 1997; Margotta et al., 1991; Hui et al., 2010; Sîrbulescu and Zupanc, 2011) in last five decades. Among them the most popular experimental protocol to study spinal cord regeneration has been tail amputation (Holtzer, 1956; Egar and Singer, 1972; Filoni et al., 1984; Margotta et al., 1991; Sîrbulescu and Zupanc, 2011). Tail amputation involves complete removal of the caudal part of tail, where muscle, skin, bone and cartilages are also removed along with spinal cord. There is complete regeneration of tail along with the spinal cord leading to functional recovery after tail amputation and a substantial progress has been made to understand the cellular mechanism of spinal cord regeneration. But there are drawbacks of using this model. The major criticism against this model is that, it does not appropriately mimic SCI in human, since there is no tail structure in humans and the nature of the injury is different.
In teleosts, the most important lesion paradigm that has been widely used to study spinal cord regeneration is complete transection (Becker et al., 1997; Goldshmit et al., 2012). Transection refers to complete severing of cord which can often lead to spinal shock in humans. It occurs rarely in comparison to hemisection which commonly occurs in gunshot wounds. Transection could be the appropriate model to study axonal regeneration since there is no axonal sparing after transection injury and some believe that axonal sparing itself could augment regeneration in mammals (Basso et al., 1996).
On the other hand, compression and crush injuries are most prevalent in mammals under experimental conditions and in human accidental injury conditions (Thuret et al., 2006). In search for an appropriate injury model to study the regeneration in teleost, we successfully established a standardized crush injury model in zebrafish, which is comparable to the mammalian mode of injury (Hui et al., 2010). Among all the experimental paradigms mentioned, standardized crush injury is the most suitable model to understand both the mammalian and the teleostean scenario compared to transection or tail amputation models. The outcome of crush injury varies when compared to transection injury, as in crush injury secondary degenerative response elicits axonal degeneration, whereas in transection injury axonal tracts are disengaged almost immediately after injury.
Materials and Reagents
- Pyrex Glass Petri dishes (150 x 20 mm) (Corning, catalog number: 3160-152BO )
- Pyrex crystallizing dish (Corning, catalog number: 3140-150 )
- Wheaton Coplin staining jars (Sigma-Aldrich, catalog number: S6016 )
- Moist tissue paper
- Needle
- Single edged surgical blade (Sigma-Aldrich, catalog number: S2771 )
- Cotton swabs, sterile (6 inch) (Medline Industries, catalog number: MDS202000Z )
- Plastic Pasteur pipettes (BRAND, catalog number: 747755 )
- Phosphate buffered saline (PBS), pH 7.4 (Sigma-Aldrich, catalog number: P4417 )
- Tricaine (MS222) (Sigma-Aldrich, catalog number: E10521 )
- Zebrafish Aquarium System water
- 0.1% cresyl violet solution (Sigma-Aldrich, catalog number: C5042 )
- 0.1% luxol fast blue solution (Sigma-Aldrich, catalog number: L0294 )
- 4% paraformaldehyde (Sigma-Aldrich, catalog number: 158127 )
- 0.5 M EDTA (Sigma-Aldrich, catalog number: E9884 )
- Paraffin (Sigma-Aldrich, catalog number: 327212 )
- Xylene (Sigma-Aldrich, catalog number: 534056 )
- 0.05% lithium carbonate solution (Sigma-Aldrich, catalog number: 255823 )
- Graded ethanol (Sigma-Aldrich, catalog number: 24102 )
Note: This product has been discontinued. - Glacial acetic acid (Sigma-Aldrich, catalog number: A6283 )
Equipment
- Leica rotary microtome (Leica Biosystems Nussloch, model: RM 2125RTS )
- Student Dumont #5 forceps (Sigma-Aldrich, catalog number: F6521 )
- Micro dissectingspring scissors (Harvard Apparatus, catalog number: 728500 )
- Stereozoom dissecting microscope (Olympus, models: SZX7 and SZ51 )
Procedure
Note: The present study was carried out according to the guidelines provided by CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environments and Forests, Government of India). Surgery protocol was approved by the Institutional Animal Ethics Committee of our department under registration with CPCSEA (CPCSEA/ORG/CH/REG NO.925/295).
- Maintenance of fish
Zebrafish stock population is either bred in our animal house facility or obtained from local pet shop. Fish are kept in separate groups of 15 in the aquatic system maintained at 28 °C on a 14 h light and 10 h dark cycle. - Preparation of surgical plates
106 mm glass Petri dish is used and moist tissue paper is placed in it, where the anesthetized fish is laid laterally (Figure 1a; Video 1). The gill is covered with tissue paper soaked in water or PBS. - Fishes of same size and age (approximately 3-4 cm in length, 4-6 months old) are anaesthetized by dipping them in 0.02% tricaine (MS222) for 3-5 min (long exposure like 10 min or more could affect post-surgical recovery of spinal cord injured fish) at room temperature. It is important to check that fish are completely anaesthetized prior to surgery. After placing the anaesthetized fish on the Petri dish, the body is touched with a needle and absence of twitching movement confirms complete anaesthesia. Petri dish with anaesthetized fish is placed under stereozoom dissecting microscope and surgery is performed under the microscope.
- Surgery protocol
- Crush injury:
- A longitudinal incision is given with a single-edged blade on the second stripe of the fish body at the level of the dorsal fin, which corresponds to the 15/16th vertebrae (Figure 1b; Video 1).
- After making the cutaneous wound (Figure 1c; Video 1), one has to scrape muscle sidewise to reach the vertebral column, which resides at a much deeper level. Blood coming to the wound site can be cleared by using a sterile cotton swab (Figure1d; Video 1).
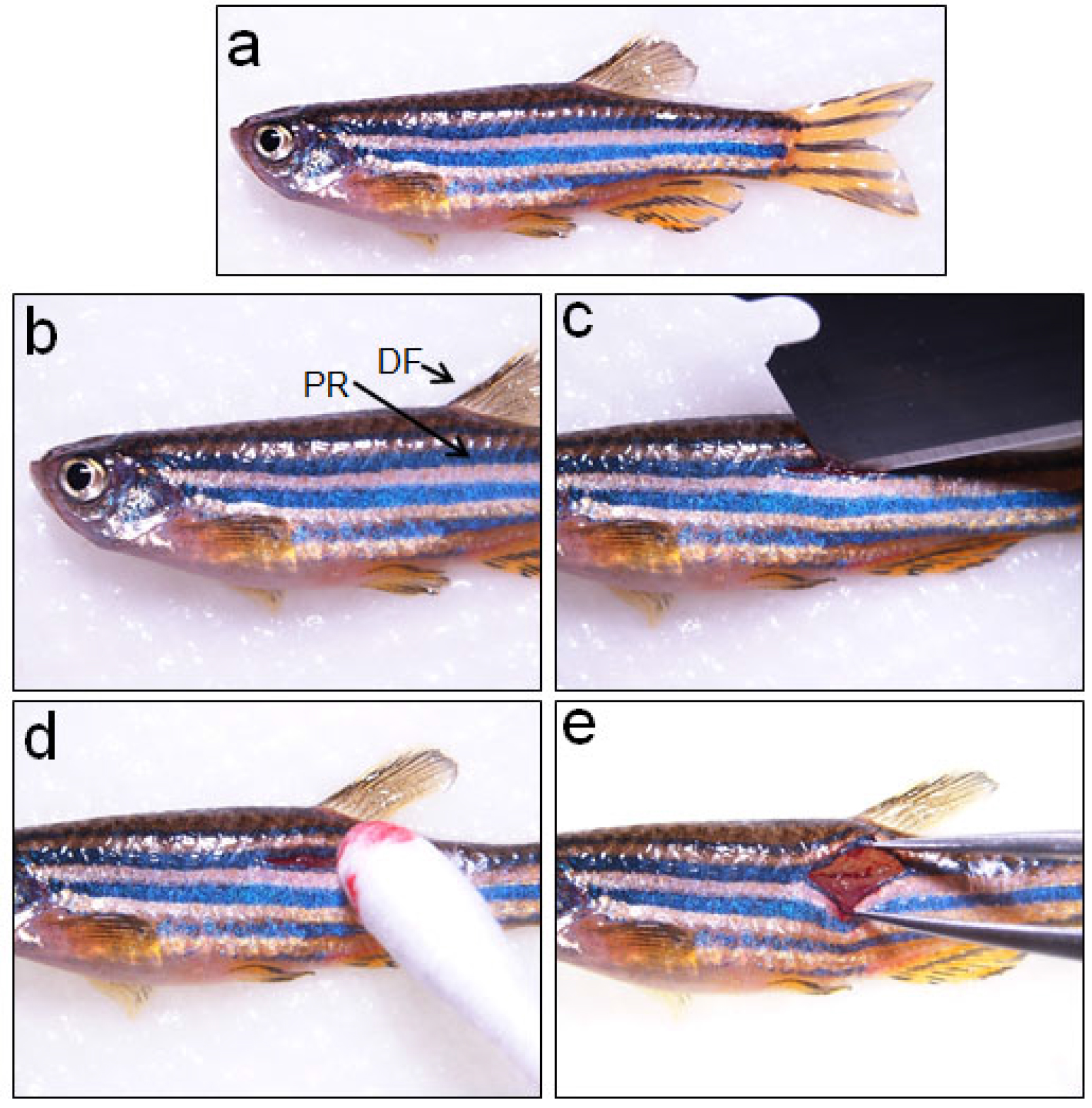
Figure 1. Making a cutaneous wound before spinal cord injury. a. An anaesthetized adult zebrafish is placed laterally over a moist tissue paper before inflicting spinal cord injury; b. The pigmented stripped region (PR) below dorsal fin (DF) is the area where the cutaneous wound is made; c. Making a cutaneous wound by a sharp blade; d. Clearing the blood from wound area by using a sterile cotton swab; e. Tearing the muscles deep inside the wound using forceps to reach and clearly visualize the spinal cord.Video 1. Inflicting spinal cord injury in adult zebrafish - Vertebral column is a hard but translucent tissue and spinal cord, encased within the vertebrae, is visible as the meninges are pigmented. After clearing the surrounding tissues, vertebral column is crushed dorso-ventrally with Dumont forceps #5 for 1 sec (Video 1; Figures 2 and 4, where the injury epicentre is marked with an asterisk).
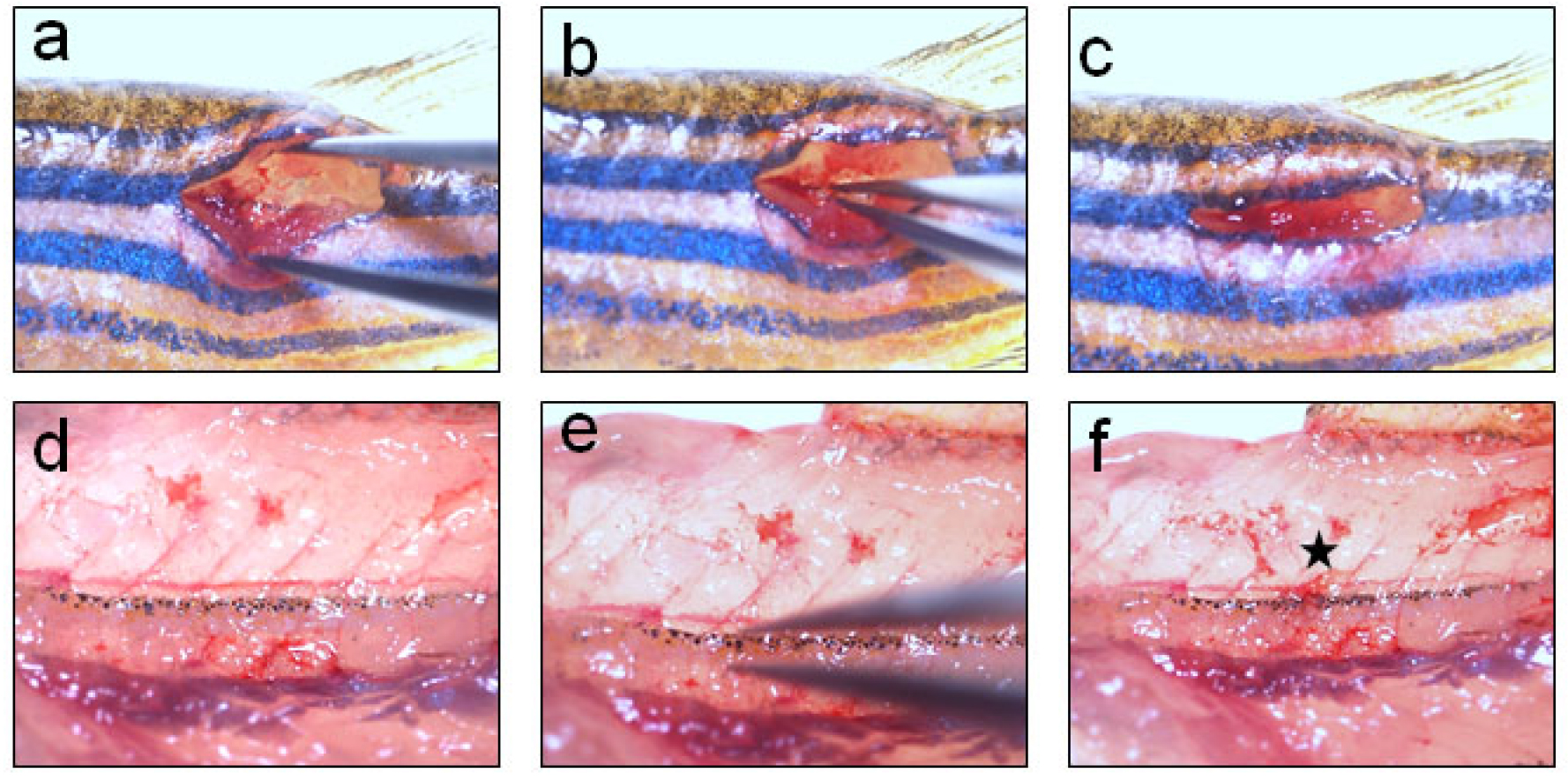
Figure 2. Crush injury to spinal cord using Dumont #5 forceps. a. Holding the forceps in stretch to clearly visualize the spinal cord before performing injury; b. Inflicting crush injury by holding the full spinal cord dorso-ventrally using Dumont #5 forceps for 1 sec; c. Covering the vertebral wound with muscle and skin; d. Higher magnification view of spinal cord inside the wound before injury; e. Higher magnification view of the spinal cord at the wound site during crush injury; f. Higher magnification view of the spinal cord inside the wound after crush injury. Star indicates the injury epicentre. - One can use the bony projections of vertebral column and mark the corresponding fin rays of dorsal fin as a land mark. We cannot perform laminectomy in zebrafish spine, as we do while inflicting SCI in mouse, because the vertebral column is too thin here. After completing injury, surrounding muscle tissues are placed back to cover the injury site and only a single suture can be given through skin to reduce the wound size.
- Fishes are returned into shallow water in a Petri dish and water is gently blown over the gills using a plastic pipette so that fish can recover from anaesthesia quickly and can swim on their own. Fishes are finally transferred to larger tanks and allowed to regenerate for a specified period of time at 28 °C.
- Transection injury
- Similar to crush injury a longitudinal incision is given on the skin laterally with a single-edged blade on the second stripe of the fish body at the level of dorsal fin, which corresponds to the 15/16th vertebrae.
- After carefully scrapping the muscle beneath the wound, spinal cord encased in the vertebral column can be visualised clearly. On reaching the vertebral column deep to the wound site, the spinal cord is completely transected using a micro-dissecting spring scissors (Figures 3 and 4).
- As spinal cord is a soft spongy tissue, a sharp cut through the gap between the two vertebrae by the two blades of micro-scissors is enough to ensure complete transection separating the rostral and caudal stumps completely from each other. The injury epicentre is marked with an asterisk in Figure 4c. The injury would lead to paralysis of posterior part of the body. After completing injury procedures, surrounding muscle tissues are placed back to cover the vertebral wound. The suture may be given through the skin (Figure S1) or tissue glue can be used (optional) to reduce the wound size. Fishes are returned to a shallow water tank and water is blown gently over the gills using a plastic pipette so that fish can recover from anaesthesia quickly.
- Fishes are finally transferred to larger tanks and allowed to regenerate for a specified period of time at 28 °C.
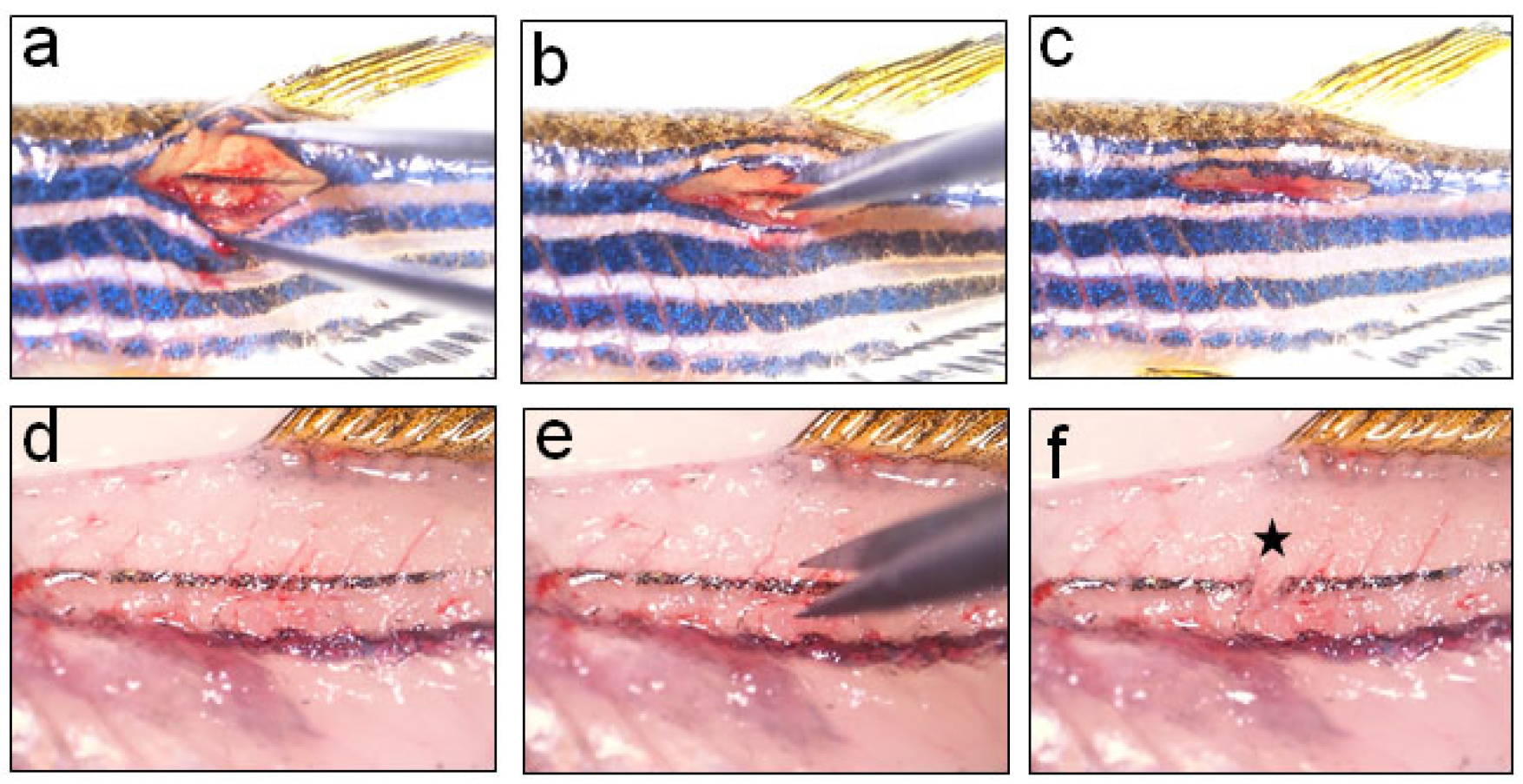
Figure 3. Transection injury to spinal cord using a micro-scissor. a. Holding the forceps in open position to clearly visualize the spinal cord before inflicting injury; b. Performing transection injury by completely severing the spinal cord into rostral and caudal stumps by using fine spring scissors; c. Covering the vertebral wound with muscle and skin; d. Higher magnification view of the spinal cord before injury; e. Higher magnification view of the spinal cord during transection injury; f. Higher magnification view of the spinal cord after transection injury. Star indicates the injury epicentre.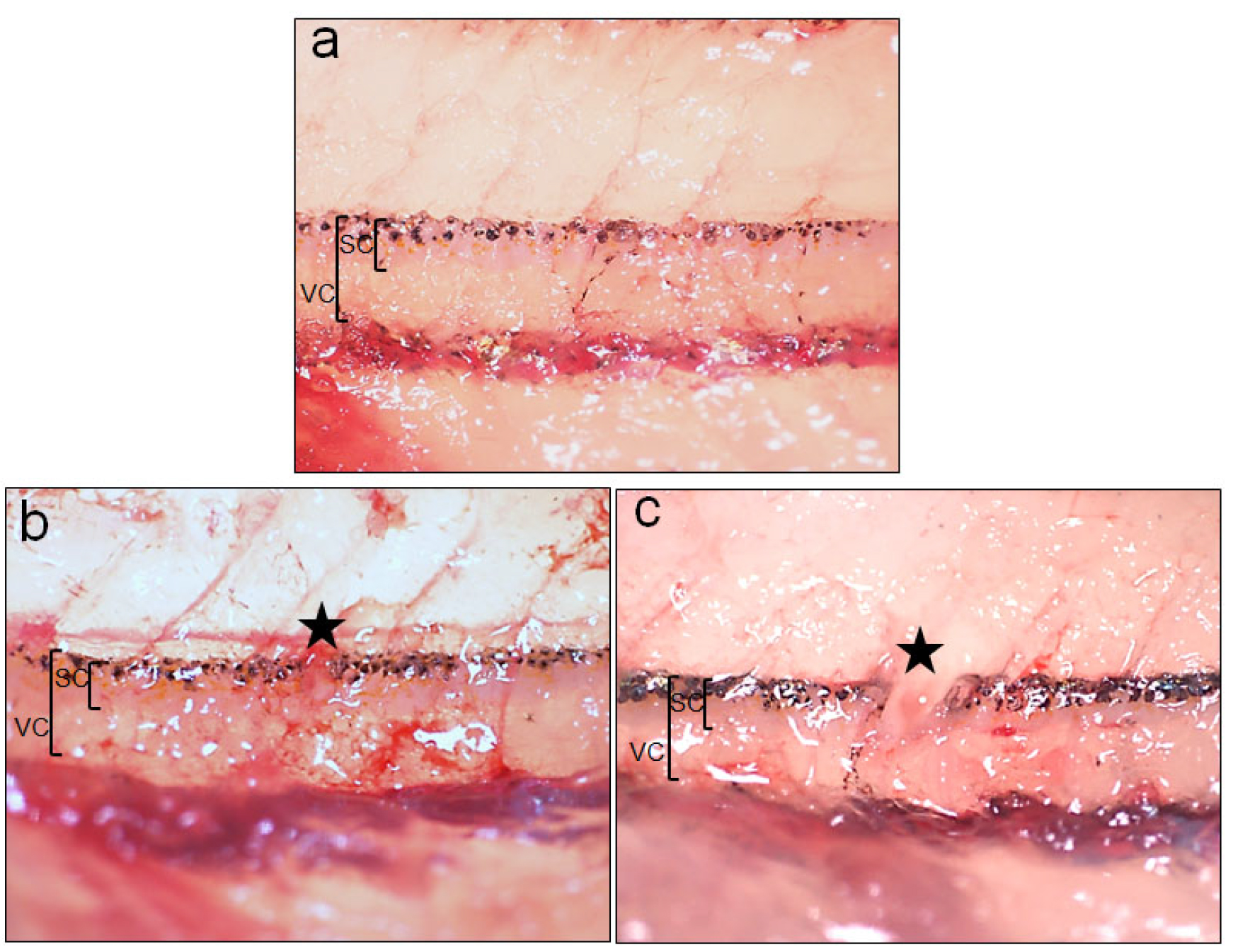
Figure 4. Uninjured and injured spinal cord after crush and transection injury. a. Higher magnification picture of the uninjured spinal cord. b. Higher magnification picture of the spinal cord after crush injury. c. Higher magnification picture of the spinal cord after transection injury. Star indicates the injured region. SC = Spinal cord, VC = Vertebral column. - Histological analysis to study time course after injury
Luxol fast blue and cresyl violet staining (0.05% lithium carbonate solution and glacial acetic acid are used for differentiation step) is performed on the spinal cord tissue sections according to the standard Klüver-Barrera protocol (Sheehan and Hrapchak, 1980). Both uninjured and injured fishes are dissected, spinal cord taken out, fixed in 4% paraformaldehyde, decalcified in 0.5 M EDTA, passed through graded alcohol and xylene and embedded in paraffin (Hui et al., 2010). Time course analysis of stained section in uninjured cord shows relative distribution of white matter and grey matter as depicted in Figure 5a. Anatomical distribution of neurons in the subependymal and axons and cell bodies in white matter are obvious. Details of the cellular components are discussed in our previous publication (Hui et al., 2010). Here substantial tissue loss is shown at the early stages after crush injury (Figure 5b) with some tissue sparing, a characteristic feature of the crush injury model. Time course cellular response depends on the degree and extent of injury. In this case, as the transection injury is very clean, not much tissue has been lost and the stump tissues are very closely apposed (Figure 5c). Regeneration is very impressive and the time frame does not vary much when compared with crush injury.
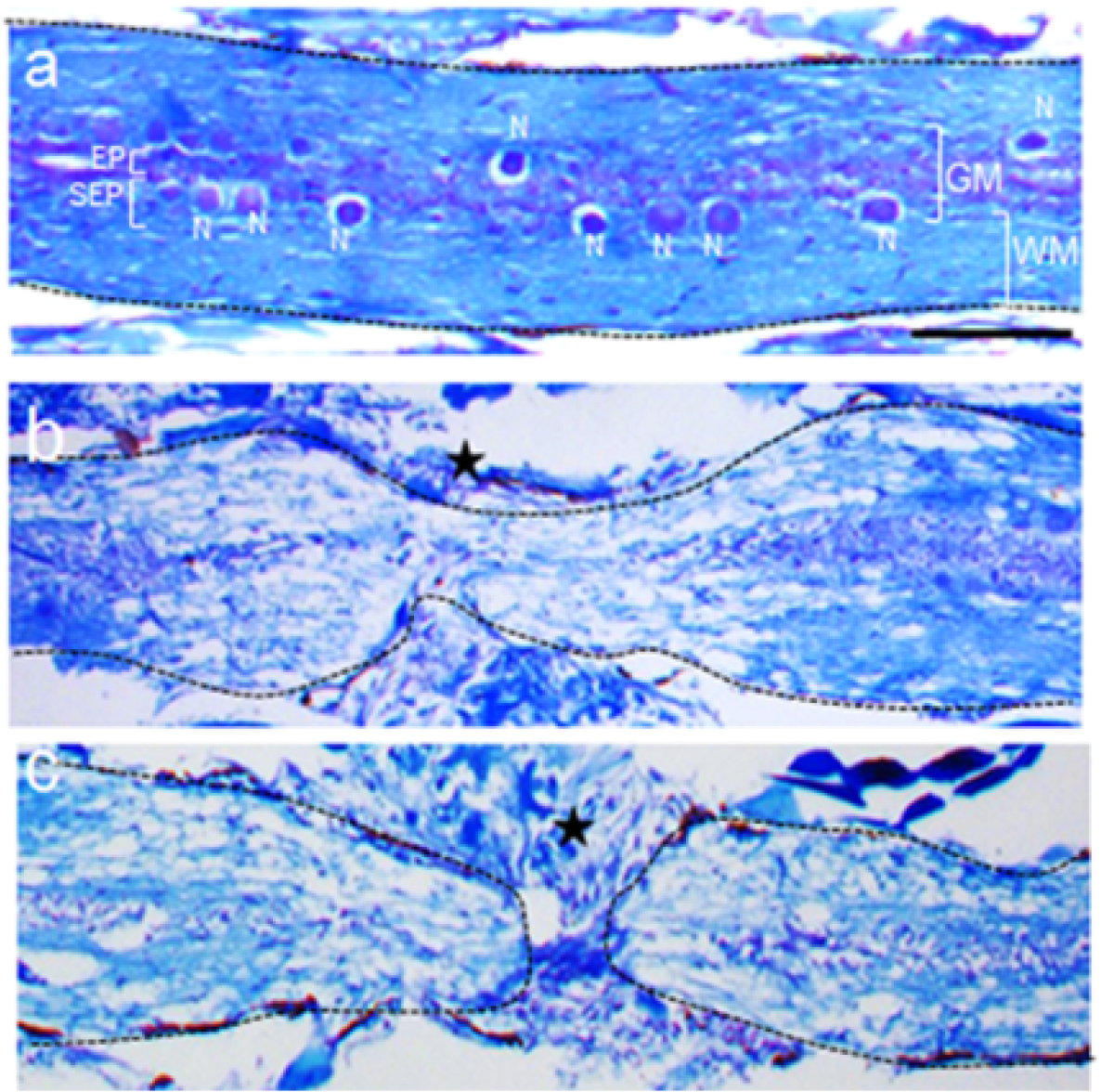
Figure 5. Luxol fast blue staining in spinal cord tissue. Histological sections of uninjured spinal cord (a), spinal cord 3 days after crush injury (b) and spinal cord 3 days after transection injury (c) stained with luxol fast blue and cresyl violet. Please note that there are some spared tissues at the injury epicentre in b, whereas two separate stump tissues are clearly visible in c, with no axonal sparing. GM = Grey matter region of spinal cord, WM = white matter region of spinal cord, N = neuron, SEP = sub-ependyma, EP = ependyma. Star indicates the approximate injury epicentre. Scale bar=100 µm (a-c).
Data analysis
Injury protocols were standardized in our laboratory where hundreds of animals have been used for our array analysis (Hui et al., 2014). We could injure 30-40 animals in a day. All the histological analysis with the spinal cord tissues were repeated 3-5 times. The pictures shown here are not repeated in other publications. However, time course analysis of regeneration in zebrafish cord was previously published (Hui et al., 2010, http://www.suklaghosh.com).
Notes
- Two very simple yet standardized protocols for inflicting crush and transection injury in adult zebrafish cord have been described. The reproducibility of these injury modalities can be followed by analysing the tissue sections stained with different staining protocols like luxol fast blue and cresyl violet or Mallory’s trichrome staining or even routine hematoxylin/eosin staining.
- Staining, particularly using longitudinal section of the spinal cord tissue, enable us to characterize the nature of injury and visualize cellular response(s) at the injury epicentre and the adjoining area after injury and compare with the normal part of the same cord.
Acknowledgments
Both these injury protocols described here are generated at Sukla Ghosh’s laboratory, Department of Biophysics, Molecular Biology and Bioinformatics, University of Calcutta, Kolkata, India. Grant support received for the work was from DBT (Govt. of India, BT/PR5489/AAQ/03/245/2004). Dr. Subhra Prakash Hui was recipient of Senior Research Fellowship from CSIR (Govt. of India). The spinal cord crush injury protocol in zebrafish has been adapted from our previous publications (Hui et al., 2010; 2014).
References
- Basso, D. M., Beattie, M. S. and Bresnahan, J. C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139(2): 244-256.
- Becker, T., Wullimann, M. F., Becker, C. G., Bernhardt, R. R. and Schachner, M. (1997). Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol 377(4): 577-595.
- Egar, M. and Singer, M. (1972). The role of ependyma in spinal cord regeneration in the urodele, Triturus. Exp Neurol 37(2): 422-430.
- Filoni, S., Bosco, L. and Cioni, C. (1984). Reconstitution of the spinal cord after ablation in larval Xenopuslaevis. Acta Embryol Morphol Exp 5(2): 109-129.
- Goldshmit, Y., Sztal, T. E., Jusuf, P. R., Hall, T. E., Nguyen-Chi, M. and Currie, P. D. (2012). Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J Neurosci 32(22): 7477-7492.
- Holtzer, S.W. (1956). The inductive activity of the spinal cord in urodele tail regeneration. J Morphol 99: 1-39.
- Hui, S. P., Dutta, A. and Ghosh, S. (2010). Cellular response after crush injury in adult zebrafish spinal cord. Dev Dyn 239(11): 2962-2979.
- Hui, S. P., Sengupta, D., Lee, S. G., Sen, T., Kundu, S., Mathavan, S. and Ghosh, S. (2014). Genome wide expression profiling during spinal cord regeneration identifies comprehensive cellular responses in zebrafish. PLoS One 9(1): e84212.
- Margotta, V., Fonti, R., Palladini, G., Filoni, S. and Lauro, G. M. (1991). Transient expression of glial-fibrillary acidic protein (GFAP) in the ependyma of the regenerating spinal cord in adult newts. J Hirnforsch 32(4): 485-490.
- Sheehan, D. C. and Hrapchak, B. B. (1980).Theory and practice of histotechnology. Battelle.
- Sîrbulescu, R. F. and Zupanc, G. K. (2011). Spinal cord repair in regeneration-competent vertebrates: adult teleost fish as a model system. Brain Res Rev 67(1-2): 73-93.
- Thuret, S., Moon, L. D. and Gage, F. H. (2006). Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7(8): 628-643.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hui, S. P. and Ghosh, S. (2016). Various Modes of Spinal Cord Injury to Study Regeneration in Adult Zebrafish. Bio-protocol 6(23): e2043. DOI: 10.21769/BioProtoc.2043.
- Hui, S. P., Sengupta, D., Lee, S. G., Sen, T., Kundu, S., Mathavan, S. and Ghosh, S. (2014). Genome wide expression profiling during spinal cord regeneration identifies comprehensive cellular responses in zebrafish. PLoS One 9(1): e84212.
Category
Neuroscience > Nervous system disorders > Animal model
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











