- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Model of Dengue Virus Infection with Serotypes 1 and 2 Clinical Isolates
Published: Vol 6, Iss 23, Dec 5, 2016 DOI: 10.21769/BioProtoc.2040 Views: 10708
Reviewed by: Yannick DebingLongping Victor TseAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Unbiased Screening of Activated Receptor Tyrosine Kinases (RTKs) in Tumor Extracts Using a Mouse Phospho-RTK Array Kit
Julian Naipauer [...] Enrique A. Mesri
Apr 20, 2019 5680 Views

Selection of Vaccinia Virus Recombinants Using CRISPR/Cas9
Anjali Gowripalan [...] David C. Tscharke
Dec 20, 2021 3417 Views
Abstract
Dengue is a global public health threat caused by infection with any of the 4 related dengue virus serotypes (DENV1-4). Clinical manifestations range from self-limiting febrile illness, known as dengue fever (DF), to life-threatening severe diseases, such as dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS). Most cases of DHF/DSS are associated with secondary heterotypic infections through a phenomenon that is described as antibody-dependent enhancement of infection (ADE). There are an estimated 400 million human infections and several hundred thousand cases of severe dengue occurring yearly. At present, however, there are no approved antiviral drugs against DENV infection. The lack of a suitable animal model has hampered the evaluation of novel antiviral candidates for DENV infection. Since DENV poorly establishes infection in immunocompetent mice, AG129 mice (lacking type I and II IFN [interferon] receptors) and mouse-adapted DENV2 strains have been applied to dengue animal models that enable to reproduce several of the major pathologies of human infection. Recently, we developed new mouse models with clinical isolates DENV1 and DENV2 that would be useful for drug testing and dengue pathogenesis studies (Watanabe et al., 2016). Here we describe the details to establish dengue mouse models of clinical isolates; from in vitro preparation of the materials to in vivo virus infection. Of note, since infectivity of DENV in mice differs among virus strains, not all clinical isolates can induce severe dengue.
Keywords: Dengue virusBackground
To overcome the drawback that DENV does not replicate well in rodent cells, many efforts have been made over the years to develop small animal models that mimic human dengue infection. The inbred mouse model system allows experimental variability to be minimized, and genetically engineered mouse models enable to reproduce some aspects of dengue clinical symptoms in the animals. A past study showed that AG129 mice (lacking type I and II IFN receptors) infected with a DENV2 clinical isolate succumbed to infection with signs of paralysis, a condition of central nervous system involvement that is rare in human cases (Shresta et al., 2004). Alternatively, mouse-adapted DENV2 strains that can induce human DHF/DSS-like diseases in AG129 mice were generated and have been used for dengue research (Shresta et al., 2006; Zellweger et al., 2010). Although the use of the mouse-adapted strains is valuable for some aspects of DENV pathogenesis studies and potential therapeutic drug testing, one considerable limitation is the variable pathogenesis depending on the serotype/genotype, and adaptation of virus in mouse might alter tissue tropism. Recently, we developed new mouse models of clinical isolates DENV1 and DENV2; the mice succumbed to infection with signs of severe dengue symptoms (Watanabe et al., 2016). Non-lethal infection with clinical isolates becomes lethal accompanied with high levels of viremia and cytokine production in the presence of DENV antibodies (Abs) (ADE condition) in AG129 mice, suggesting that this system enables to extend the use of clinical DENV isolates for the study of Ab-mediated DENV pathogenesis and the evaluation of anti-dengue candidates.
Materials and Reagents
- 50 ml centrifuge tubes (Corning, Falcon®, catalog number: 357550 )
- 0.45 μm membrane filter (Sartorius, Minisart®, catalog number: 16537 )
- 0.2 μm membrane filter unit for bulk culture (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 567-0020 )
- HiTrap Protein G HP-5 ml (GE Healthcare, catalog number: 170-0405-01 )
- 96-well PCR plate (Bio-Rad Laboratories, catalog number: HSP9601 )
- SnakeskinTM dialysis tubing 10 kDa (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 68100 )
- 30 G insulin syringe (BD, catalog number: 328818 )
- 27 G needle (BD, catalog number: 305109 )
- 1.5 ml Eppendorf tubes (Corning, Axygen, catalog number: MCT-150-c )
- Plate cover seal (Bio-Rad Laboratories, catalog number: MSB1001 )
- CryoTubes vials for freezing viruses (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 368632 )
- Dengue virus: DENV-2 mouse-adapted S221 strain (Zellweger et al., 2010), DENV-1 clinical isolate (EDEN1: GenBank accession
EU081230.1) (Low et al., 2006), DENV2 clinical isolate (EDEN2: GenBank accession EU081177.1) (Low et al., 2006) - Cells of the Aedes albopictus C6/36 line (clone C6/36) (ATCC, catalog number: CRL-1660TM )
- Cells of the baby hamster kidney cell line (BHK-21 [C-13]) (ATCC, catalog number: CCL-10TM )
- Hybridoma cells (D1-4G2-15) (ATCC, catalog number: HB-112TM )
- Sv/129 mice deficient in type I and II IFN receptors (AG129 mice) (B&K Universal)
- RPMI1640 medium (Thermo Fisher Scientific, GibcoTM, catalog number: 11875093 )
- Heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10082147 )
- Liquid nitrogen
- 3.7% formaldehyde (Sigma-Aldrich, catalog number: F1635 )
- Crystal violet (Sigma-Aldrich, catalog number: C3886 )
Note: This product has been discontinued. - Protein-Free hybridoma medium (PFHM-II medium) (Thermo Fisher Scientific, GibcoTM, catalog number: 12040077 )
- 1x PBS (Lonza, catalog number: 17-516Q )
- Glycine (Sigma-Aldrich, catalog number: G7126 )
- 0.25% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Ethylenediaminetetraacetic acid (EDTA) (EMD Millipore, catalog number: 819040 )
- QIAamp Viral RNA Mini Kit (Qiagen, catalog number: 52906 )
- qScript One-Step qRT-PCR Kit (Quantabio, catalog number: 95057 )
- Methyl-cellulose powder (EMD Millipore, catalog number: 17851 )
- L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081 )
- Penicillin and streptomycin (PenStrep) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- 7.5% sodium bicarbonate solution (Thermo Fisher Scientific, GibcoTM, catalog number: 25080094 )
- 1 M HEPES (Thermo Fisher Scientific, GibcoTM, catalog number: 15630080 )
- Ethanol (EtOH) (EMD Millipore, catalog number: 1009832511 )
- RPMI 1640 powder (Thermo Fisher Scientific, GibcoTM, catalog number: 31800022 )
- 1 N HCl (Sigma-Aldrich, catalog number: 258148 )
- Tris (First BASE Laboratories Sdn Bhd, catalog number: BIO-1400 )
- Primers and probes
DENV1 forward primer: 5’-ACACCAGGGGCTGTACCTTGG-3’
DENV1 reverse primer: 5’-CATTCCATTTTCTGGCGTTCT-3’
DENV1 taqman probe: FAM-5’-CTGTCTCTACAGCATCATTCCAGGCA-3’-TAMRA
DENV2 forward primer: 5’-CATATTGACGCTGGGAAAGA-3’
DENV2 reverse primer: 5’-AGAACCTGTTGATTCAAC-3’
DENV2 taqman probe: FAM-5’-CTGTCTCCTCAGCATCATTCCAGGCA-3’-TAMRA - Standard for realtime RT-PCR: plasmids containing whole genome sequences of DENV-1 (EDEN1: EU081230.1) or DENV-2 (EDEN2: EU081177.1)
- 0.8% methyl-cellulose medium (see Recipes)
- 1% Crystal violet (see Recipes)
- 0.1 M glycine (pH 2.7) (see Recipes)
- 1 M Tris- HCl (pH 9.0) (see Recipes)
Equipment
- NuncTM 175 cm2 angled-neck easy flasks (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 159920 )
- Cell scraper (Corning, catalog number: 3010 )
- Incubator (SANYO, model: MIR-262 ) without CO2 atmosphere at 28 °C
- Humidified incubator (NuAire, model: NU5500 ) with 5% CO2 atmosphere at 37 °C
- Swinging rotor centrifuge (for cells) (Thermo Fisher Scientific, model: Heraeus Multifuge 3S-R )
- Benchtop fixed-angle rotor centrifuge (for serum) (Eppendorf, model: 5424 )
- -80 °C freezer (Thermo Fisher Scientific, Thermo ScientificTM, model: Forma 900 Series )
- Autoclave (TOMY DIGITAL BIOLOGY, model: SX-700 )
- AKTApurifierTM UPC 10 (GE Healthcare, catalog number: 28406268 )
- pH meter (Sartorius, model: pH Basic Series )
- NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: ND-2000 )
- Real-time thermal cycler (Bio-Rad Laboratories, model: CFX96 )
- Mouse restrainer (Plas-labs, catalog number: 551-BSRR )
- Olympus inverted fluorescence microscope (Olympus, model: IX71 )
Software
- GraphPad Prism software
Procedure
- Generation of DENV stocks
Note: A high virus titer of clinical DENV strains is required to induce severe diseases in AG129 mice. The virus titer obtained from C6/36 cells differs among virus strains. Thus, optimization of culture condition such as multiplicity of infection (MOI) of virus inoculum or timing of virus collection is needed to obtain enough virus titer for each virus strain.- Maintain C6/36 cells in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and streptomycin and 25 mM HEPES. Incubate cells at 28 °C under non CO2 atmosphere condition.
Note: Growth media supplemented with 20% FBS improves cell growth if C6/36 cells do not proliferate desirably. - Grow C6/36 cells in a 175 cm2 flask in culture medium until ~90% confluency (see Figure 1).
- Thaw virus stock and dilute with serum-free RPMI 1640 medium. Discard medium from culture flask and add 5 ml of virus inoculum at MOI of 0.1 (for EDEN1 or S221) or 1 (for EDEN2) into 175 cm2 flask.
Note: At confluency, there are approximately 3 x 107 cells in a T175 flask, hence for an MOI 0.1-1, 3 x 106-3 x 107 pfu are needed (refer to Procedure B on how to determine virus titers). - Incubate for 1 h at 28 °C under non CO2 atmosphere condition.
- Remove virus inoculum and add 25 ml RPMI 1640 medium (2% FBS) to the 175 cm2 flask.
- Incubate for 4-7 days at 28 °C under non CO2 atmosphere condition.
Note: Cytopathic effect is observed depending on virus strain (seen in EDEN2 infection but not in EDEN1 or S221 infection). Check virus titer in the supernatant at different time point to determine the optimal day to obtain high virus titer (refer to Procedure B on how to determine virus titers). Incubation periods are 5 days for EDEN2 and S221, and 7 days for EDEN1. - Scrape cells and transfer into a centrifuge tube, then spin down the cells at 1,800 x g for 10 min at 4 °C.
- Collect supernatant and transfer into a fresh tube through a 0.45 μm membrane filter.
- Aliquot virus into cryotubes and store in a -80 °C freezer or liquid nitrogen until use.
Note: It is recommended to store virus stock in liquid nitrogen for long-term storage.
- Maintain C6/36 cells in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and streptomycin and 25 mM HEPES. Incubate cells at 28 °C under non CO2 atmosphere condition.
- Determination of viral titer by plaque assay
- Maintain BHK-21 cells in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine and 100 U/ml penicillin and streptomycin in a humidified incubator with 5% CO2 atmosphere at 37 °C.
- Seed cells at 2 x 105 cells per well in 500 μl into 24-well plate.
- Incubate cells overnight at 37 °C in 5% CO2 incubator to allow cells to adhere.
Note: Cells become confluent on the following day. Two days incubation after seeding of 7 x 104 cells is also viable. - Prepare 10-fold serial dilutions of virus in serum-free RPMI 1640.
- Remove culture supernatant of BHK-21 cells and add 200 μl of diluted virus into each well.
Note: Virus should be added immediately after removing culture supernatant to avoid cells drying out. - Incubate the plate for exactly 1 h at 37 °C in 5% CO2 incubator.
- Remove virus and add 500 μl of 0.8% methyl-cellulose medium supplemented with 2% FBS.
- Incubate plate for 4-5 days at 37 °C in 5% CO2 incubator.
Note: The plaque size is affected by virus replication rate. Check the plaque size visually before fixation to obtain clear plaque morphology. Plates of EDEN1 infection are normally incubated until day 4 post-infection, whereas plates of EDEN2 or S221 are usually incubated until day 5 post-infection. - Overlay 1 ml of 3.7% formaldehyde onto the cells and fix for 20 min at room temperature.
Note: Alternatively, the plates can be put into a hermetic container containing 5 L of 3.7% formaldehyde and fixed for 20 min without removing methyl-cellulose medium. The formaldehyde solution can be kept for 3-4 months, however, longer time fixation times may be required if the 3.7% formaldehyde is not fresh. Once the solution turns brown and turbid, replace it. - Rinse the plate with copious volume of water in a container. Shake the plate robustly to remove methyl-cellulose medium completely.
- Add 1-3 drops of 1% crystal violet into each well and stain for 2 min.
- Rinse the plate with copious volume of water in a container and shake the plate to remove excess water.
- Dry the plate out and count the number of plaques to determine virus titer.
Caluculation of plaque forming units (pfu):
Virus titer (pfu/ml) = average number of plaques/200 μl inoculum x 1,000 μl x dilution factor
- Maintain BHK-21 cells in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine and 100 U/ml penicillin and streptomycin in a humidified incubator with 5% CO2 atmosphere at 37 °C.
- Preparation of α-DENV E protein antibodies (4G2) from hybridoma cells
- Culture 4G2 hybridoma cells in 50 ml of PFHM-II (Protein-Free hybridoma medium) in 175 cm2 flasks in humidified incubator with 5% CO2 atmosphere at 37 °C.
Note: It is recommended to culture the cells in RPMI 1640 (10%) during the initial period of several days after thawing cells. Once the cells grow well, replace the media gradually by increasing the proportion of PFHM-II media in RPMI 1640 from 10% (vol/vol) to finally 100% PFHM-II media (example: 10%, 25%, 50%, 75% and 100%). - Collect cell suspension into a centrifugal tube when the cells become confluent (the color of culture media turns to orange or yellow).
- Centrifuge cells at 900 x g for 5 min at room temperature.
- Collect the supernatant and stored at 4 °C without filtration until enough amount of supernatant is obtained.
- Continue culturing the cells and repeat steps C2-C4 if a large amount of the supernatant is required.
- Filter the supernatant through a 0.2 μm membrane filter unit.
- Load the 4G2 supernatant onto a 5 ml Protein G column pre-equilibrated in pH 7.2 PBS.
Note: 4G2 antibody is purified using the AKTApurifier. Refer to the manufacturer’s instruction guides regarding sample loading specifications. - Wash the column with PBS using 5x the column volume (i.e., 25 ml).
- Prepare a 96-well block containing 60 μl 1 M Tris-HCl.
Note: The standard ratio of Tris-HCl to glycine (100:6) for neutralization is subjected to change depending on the concentration of buffers prepared. Volume of Tris-HCl to be added required for neutralization (pH 7) can be adjusted by pH paper testing. - Elute antibodies using 100% 0.1 M glycine and collect 1 ml fractions into the wells of the block.
Note: Check the purity of the antibody by running a SDS-PAGE. - Select fractions of high purity and collect into a dialysis membrane, and then dialyse against PBS overnight.
- Quantitate the concentration of the purified antibody using NanoDrop.
- Culture 4G2 hybridoma cells in 50 ml of PFHM-II (Protein-Free hybridoma medium) in 175 cm2 flasks in humidified incubator with 5% CO2 atmosphere at 37 °C.
- DENV infection in AG129 mice and measurement of viremia
Note: Infectivity of DENV in AG129 mice differs among virus strains. DENV2 clinical strains are generally more infectious than DENV1 clinical strains in the mice (our unpublished observation). Infection in the presence of DENV-specific antibodies (ADE condition) can enhance the infection level and make it possible to induce mortality. Here we describe the methods for two successful mouse models of clinical isolates of DENV1 (EDEN1 strain) and DENV2 (EDEN2 strain) (Watanabe et al., 2016) together with the pioneering mouse model using mouse-adapted DENV2 (S221 strain) (Zellweger et al., 2010).- Prepare AG129 mice aged 7-11 weeks.
Note: AG129 mice were purchased from B&K Universal (UK) and the breeding pairs were maintained under specific pathogen-free (SPF) conditions in the animal facility. One female AG129 mouse bears approximately 4 pups per month, therefore, maintainance of 10-breeding pairs produce about 40 mice per month. - Dilute purified 4G2 with PBS to concentrations of 50 μg/100 μl for EDEN1 or EDEN2 infection and 10 μg/100 μl for S221 infection.
- Administer 100 μl of diluted 4G2 into AG129 mice intraperitoneally using a 30 G insulin syringe one day prior to virus infection.
- Dilute virus stock with PBS to concentrations of 7 x 107 pfu/200 μl for EDEN1, 1 x 108 pfu/200 μl for EDEN2 and 2 x 104 pfu/200 μl for S221. Keep the diluted virus on ice.
Note: Inoculation of these virus titer induces lethal infection in the presence of 4G2 antibodies (ADE condition) but not in the absence of 4G2. - Hold a mouse steadily in a mouse restrainer. Inoculate 200 μl of virus inoculum intravenously into mice through the tail vein using 30 G insulin syringe.
Note:Up to 1 ml of virus can be inoculated intravenouslly into mice. Thus, as far as the titer of virus stock exceeds 1 x 108 pfu/ml, enough virus can be delivered into the mice for lethal infection. - Observe mouse disease status and survival rate until day 10 post-infection.
Note: Mice usually succumb to infection by day 5 post-infection accompanied with a severe form of human DHF/DSS-like diseases (Zellweger et al., 2010; Watanabe et al., 2016). - Add 10 μl of 1% (wt/vol) EDTA (in PBS) into each 1.5 ml Eppendorf tube for blood collection. Hold the mouse vertically with the head tilted back and collect approximately 100 μl of blood from facial vein using a 27 G needle (final 0.1% EDTA).
Note: It is possible to collect 100 μl of blood from each mouse on 10 consecutive days. - Centrifuge the blood samples at 13,500 x g for 3 min.
- Collect serum and store at -80 °C until use.
- Collect 20 μl of each serum sample and mix with 120 μl PBS.
- Extract RNA from serum using QIAamp Viral RNA Mini Kit according to the manufacturer’s instruction.
- Make 20 μl reaction mixture (4 μl RNA plus 16 μl PCR reagent mixture) using the qScript One-Step qRT-PCR Kit. Prepare standard curves with plasmid DNA ranging from 107 to 101 copies/4 µl. Perform quantitative RT-PCR reactions in duplicate in Bio-Rad Real-time thermal cycler CFX96 according to the manufacturer’s instruction.
- Run the qPCR using the following program

- Prepare AG129 mice aged 7-11 weeks.
Data analysis
Significant differences of viremia between data groups can be determined by a 2-tailed Student’s t-test analysis using online free software. For mouse survival, statistical analysis can be performed by the log-rank test using the GraphPad Prism software. P values less than 0.05 are considered significant.
Notes
This protocol was optimized for the two DENV clinical strains (EDEN1 and EDEN2). The protocol can be adapted to other clinical isolates including DENV serotype 3 (our unpublished data). However, not all clinical isolates can induce high mortality in AG129 mice due to their different infectivity to the mice.
Representative data
Figure 1. Bright field image of C6/36 cells showing ~90% confluency that is ready for DENV infection. The image was taken under 10x magnification using Olympus inverted fluorescence microscope.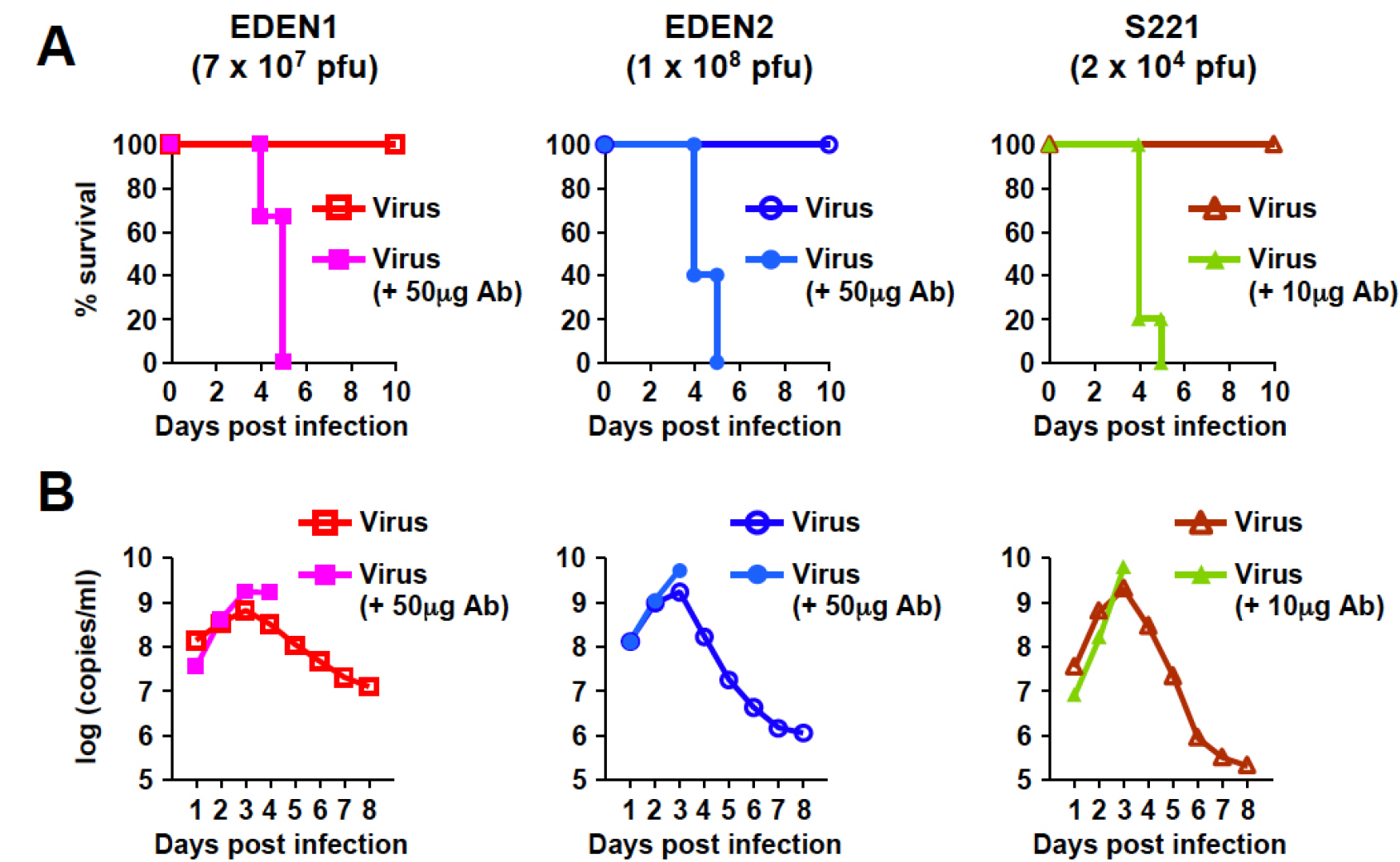
Figure 2. Infection models of EDEN1, EDEN2 and S221 in AG129 mice. AG129 mice were inoculated intravenously with EDEN1 (7 x 107 pfu), EDEN2 (1 x 108 pfu) or S221 (2 x 104 pfu) for non-lethal infection. For the lethal ADE infection, mice were pre-injected intraperitoneally with 50 μg (for EDEN1 and EDEN2) or 10 μg (for S221) 4G2 antibody one day prior to infection. A. Mouse survival rate was monitored by day 10 post-infection. B. Blood samples were collected on days 1-8 post-infection and mixed serum from each group was subjected to real-time RT-PCR to obtain the average viral genome copy numbers. Mouse number per group is 5 (EDEN1 infection) or 6 (EDEN2 and S221 infection).
Note: Inoculation of virus alone does not induce mortality (A), whereas inoculation of virus in the presence of 4G2 causes 100% mortality (A) accompanied with enhanced peak viremia levels (B). These mouse models are applicable to in vivo evaluation of antiviral agents against not only mouse-adapted dengue virus but also dengue clinical strains (Watanabe et al., 2016).
Recipes
- 0.8% methyl-cellulose medium
- Add 8 g methyl-cellulose powder into 500 ml double distilled water, autoclave twice to dissolve the powder
- Prepare 500 ml 2x RPMI 1640 media by dissolving RPMI 1640 powder in double distilled water followed by supplementing with 4% heat-inactivated FBS, 4 mM L-glutamine, 200 U/ml penicillin and streptomycin, 0.075% sodium bicarbonate solution and 50 mM HEPES
- After filtration of 2x RPMI 1640 media through 0.2 μm membrane filter unit, mix well with 500 ml prepared methyl-cellulose
- Store at 4 °C
- 1% Crystal violet
Add 5 g Crystal violet to 100 ml 100% EtOH and mix well to dissolve powder
Add 400 ml double distilled water
Store at room temperature - 0.1 M glycine (pH 2.7)
Add 3.75 g glycine into 500 ml double distilled water
Adjust the pH to 2.7 using 1 N HCl
Store the solution at 4 °C - 1 M Tris-HCl (pH 9.0)
Add 30.3 g of Tris into 250 ml double distilled water
Adjust the pH to 9.0 using 1 N HCl
Store the solution at room temperature
Acknowledgments
This work was supported by the Ministry of Health in Singapore through NMRC/MOHIAFCat1/0008/2014. The protocol described here was based on the following paper: Watanabe et al. (2016).
References
- Low, J. G., Ooi, E. E., Tolfvenstam, T., Leo, Y. S., Hibberd, M. L., Ng, L. C., Lai, Y. L., Yap, G. S., Li, C. S., Vasudevan, S. G. and Ong, A. (2006). Early Dengue infection and outcome study (EDEN) - study design and preliminary findings. Ann Acad Med Singapore 35(11): 783-789.
- Shresta, S., Kyle, J. L., Snider, H. M., Basavapatna, M., Beatty, P. R. and Harris, E. (2004). Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical.. J Virol 78(6): 2701-2710.
- Shresta, S., Sharar, K. L., Prigozhin, D. M., Beatty, P. R. and Harris, E. (2006). Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol 80(20): 10208-10217.
- Watanabe, S., Chan, K. W., Dow, G., Ooi, E. E., Low, J. G. and Vasudevan, S. G. (2016). Optimizing celgosivir therapy in mouse models of dengue virus infection of serotypes 1 and 2: The search for a window for potential therapeutic efficacy. Antiviral Res 127: 10-19.
- Zellweger, R. M., Prestwood, T. R. and Shresta, S. (2010). Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7(2): 128-139.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Watanabe, S., Chan, K. W. K. and Vasudevan, S. G. (2016). Mouse Model of Dengue Virus Infection with Serotypes 1 and 2 Clinical Isolates. Bio-protocol 6(23): e2040. DOI: 10.21769/BioProtoc.2040.
Category
Microbiology > in vivo model > Viruses
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












