- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Primary Culture of Various Cell Types from Whole Human Endometrial Biopsies
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2028 Views: 21350
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2245 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1478 Views
Abstract
The isolation and primary culture of cells from human endometrial biopsies provides valuable experimental material for reproductive and gynaecological research. Whole endometrial biopsies are collected from consenting women and digested with collagenase and DNase I to dissociate cells from the extracellular matrix. Cell populations are then isolated through culturing, filtering and magnetic separation using cell-surface antigen markers. Here we provide a comprehensive protocol on how to isolate and culture individual cell types from whole endometrial tissues for use in in vitro experiments.
Background
The human endometrium is the inner most mucosal layer of the uterus. It consists of a columnar epithelium and basal stromal layer that undergoes cyclical regeneration, growth and transformation in response to circulating hormones. The differentiation of the endometrial lining into a glandular secretory phenotype provides a hospitable environment for blastocyst implantation and successful pregnancy. In the absence of pregnancy this layer is shed, leading to menstruation. The isolation and culture of cells from human endometrial biopsies allows for in vitro functional assessment and the study of cell characteristics in relation to patient outcomes. The isolation and culture of endometrial cells is an invaluable research model to investigate many aspects of gynaecological and obstetrical medicine including infertility, implantation failure, recurrent miscarriage and menstrual disorders. Whole human endometrial biopsies contain human endometrial stromal cells (HESCs), luminal and glandular endometrial epithelial cells (HEECs), red blood cells and a mixed population of immune cells. HESCs can be easily and inexpensively isolated from whole biopsies and actively proliferate in culture for up to 5 passages without significant change in their growth dynamics. This provides a large window of opportunity for experimental analysis. Furthermore, within dissociated HESCs there is a sub-population of perivascular progenitor mesenchymal stem-like cells that can be isolated using the perivascular-specific antigen SUSD2 and its cognate antibody W5C5. Here we provide in detail an updated and expanded protocol from those published previously (Masuda et al., 2012; Chen and Roan, 2015) to describe steps in isolating and culturing different cell types from whole human endometrium. We provide further information on biopsy collection, detailed protocols for isolation of progenitor cells and additional procedures to increase epithelial cell yield and culturing efficiency.
Materials and Reagents
- Petri-dish 92 x 16 mm (SARSTEDT, catalog number: 82.1473 )
- Disposable scalpels (Swann Morton, catalog number: 0501 )
- 15 ml CELLSTAR® tubes (Greiner Bio One, catalog number: 188261 )
- 50 ml CELLSTAR® tubes (Greiner Bio One, catalog number: 227270 )
- 7 ml Bijoux tubes (Greiner Bio One, catalog number: 189176 )
- FisherBrandTM Nylon mesh cell strainer, 40 µm (Thermo Fisher Scientific, Fisher Scientific, catalog number: 11587522 )
- 0.2 µm Minisart® NML syringe filter (Sartorius Stedim Biotech, catalog number: 16534-K )
- 20 ml syringes (BD, catalog number: 300613 )
- 60 ml syringes (BD, catalog number: 309653 )
- Sterile pipette filter-tips 1,000 µl (Alpha Laboratories, catalog number: ZP1250S )
- Sterile pipette filter-tips 100 µl (Alpha Laboratories, catalog number: ZP1200S )
- FisherbrandTM glass Pasteur pipettes (Thermo Fisher Scientific, Fisher Scientific, catalog number: 1156-6963 )
- MS columns (Miltenyi Biotec, catalog number: 130-042-201 )
- Wallach Endocell® disposable endometrial cell sampler (Wallach Surgical Devices, catalog number: 908014A )
- Human endometrial biopsies (see step A)
- Cell culture media
- DMEM/F12 (1:1) with phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 31330-038 )
- L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030-081 )
- Antibiotic/antimycotic (Thermo Fisher Scientific, GibcoTM, catalog number: 15240-062 )
- β-estradiol (Sigma-Aldrich, catalog number: E2758 )
- Recombinant human insulin (Sigma-Aldrich, catalog number: 91077C )
- Acetic acid, glacial ≥ 99.7% (Sigma-Aldrich, catalog number: 695092 )
- DMEM/F12 (1:1) with phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 31330-038 )
- Tissue digestion media
- DMEM/F12, phenol-free media (Thermo Fisher Scientific, GibcoTM, catalog number: 11039-021 )
- Collagenase from Clostridium histolyticum (Sigma-Aldrich, catalog number: C9891-500MG )
- DNase I from bovine pancreas (Roche Diagnostics, catalog number: 11284932001 )
- DMEM/F12, phenol-free media (Thermo Fisher Scientific, GibcoTM, catalog number: 11039-021 )
- Trypsin-EDTA, 0.25% (Thermo Fisher Scientific, GibcoTM, catalog number: 25200-056 )
- Ficoll-paque plus medium (GE Healthcare, catalog number: 17-1440-02 )
- Separation buffer (see Recipes)
- PE anti-human SUSD2, clone: W5C5 antibody (Biolegend, catalog number: 327406 )
- Anti-PE microbeads (Miltenyi Biotec, catalog number: 130-048-801 )
- Ethanol, absolute (Thermo Fisher Scientific, Fisher Scientific, catalog number: 10437341 )
- Sterile distilled water
- Dextran-coated charcoal (DCC)-treated FBS (see Recipes)
- Digestion media (see Recipes)
- Culture media (see Recipes)
Equipment
- 25 cm2 CELLSTAR® culture flasks (Greiner Bio One, catalog number: 690175 )
- 75 cm2 CELLSTAR® culture flasks (Greiner Bio One, catalog number: 658175 )
- 5 ml serological pipettes (Greiner Bio One, catalog number: 606180 )
- 10 ml serological pipettes (Greiner Bio One, catalog number: 607180 )
- Pipette controller/pipette aid (e.g., STARLABS, catalog number: S7166-0010 )
- Vacuum-driven 0.22 μm filtration system (EMD Millipore, catalog number: SCGPT05RE )
- LUNATM BF automated cell counter (Logos Biosystems, catalog number: L10001 )
- LUNATM cell counting slides (Logos Biosystems, catalog number: L12001 )
- miniMACS separator (Miltenyi Biotec, catalog number: 130-042-102 )
- MACS multistand (Miltenyi Biotec, catalog number: 130-042-303 )
- Walker Class II cell culture microbiological safety cabinet (Walkers Safety Cabinets, model: Class II MSC )
- FisherbrandTM FB 70155 aspirator (Thermo Fisher Scientific, Fisher Scientific, catalog number: 11533485 )
- Grant Instruments water bath (Grant Instruments, model: OLS200 )
- Thermo Scientific HeracellTM 150i humidified tissue culture incubator (set at 37 °C and 5% CO2) (Thermo Fisher Scientific, catalog number: 51026280 )
- Sigma 3-16KL bench-top centrifuge (Sigma Laborzentrifugen, model: 3-16KL )
- Bright-field Leica DMIL microscope (Leica Microsystems, model: Leica DMIL )
Procedure
- Collection of human endometrial biopsies
Endometrial biopsies are obtained from women attending the Implantation Clinic, a dedicated research clinic at University Hospitals Coventry and Warwickshire National Health Service Trust. All research was undertaken with full ethical approval and with written informed consent obtained from all participants in accordance with the guidelines in The Declaration of Helsinki 2000. Biopsies are taken during the secretory phase of the menstrual cycle using an Endocell cannula, starting from the uterine fundus and moving downward to the internal cervical ostium. The endometrial biopsy is placed in a labelled Bijoux tube containing 5 ml cell culture media, and processed immediately.
Notes:- Biopsy sizes vary considerably depending on the patient and clinical professional performing the procedure. The hormonal status of the patient and the stage of menstrual cycle in which biopsies are obtained will effect yielding and cell composition due to the transient phasic dynamics of the endometrium. Our biopsies are timed to the secretory phase of the menstrual cycle, but readers are encouraged to time collections around their own experimental hypothesis.
- Typically, biopsies have a uniform thickness (see Figure 1B) but can vary in length from < 1-6 cm. Tissue is also fragile and may fragment on collection or in transit, or be contaminated with blood or mucus. Readers should follow the protocol exactly in all situations. The only exception is when mucus content exceeds that of endometrial tissue. In this case the biopsy is discarded.
- Biopsy sizes vary considerably depending on the patient and clinical professional performing the procedure. The hormonal status of the patient and the stage of menstrual cycle in which biopsies are obtained will effect yielding and cell composition due to the transient phasic dynamics of the endometrium. Our biopsies are timed to the secretory phase of the menstrual cycle, but readers are encouraged to time collections around their own experimental hypothesis.
- Tissue digestion, isolation and culture of Human Endometrial Stromal Cells (HESCs)
Note: Ensure sterility. Work in a Class II microbiological safety cabinet and ensure full aseptic technique.- Pre-prepare digestion media and pre-warm to 37 °C in a water bath.
- Decant as much media as possible from the Bijoux vial into the lid of the Petri-dish without discarding tissue. Transfer biopsy and any remaining media into the Petri-dish (Figure 1A).

Figure 1. Preparation of endometrial biopsies for digestion. Collect whole biopsies in culture media and process immediately (A). Remove all media using a manual pipette (B) before dicing using a down-ward tapping motion with a sterile scalpel (C) for 5 min or until pulp-like (D). Tissue pieces are then digested for 1 h at 37 °C with collagenase and DNase I. - Manually aspirate the excess media from around the tissue using a P1000 filter tip (Figure 1B).
Note: Do not use an aspirator or risk losing tissue. - Dice the tissue with the scalpel using a downward tapping motion for at least 5 min (Figure 1C) or until large pieces have disappeared and the tissue appears pulp-like (Figure 1D).
- Add 10 ml of digestion media to the Petri-dish through a 20 ml syringe and 0.2 µm syringe filter.
- Transfer the media with the tissue fragments to a 50 ml conical tube using a 10 ml serological pipette.
- Shake the tube for 15 sec and incubate at 37 °C for 1 h. Shake for 15 sec at 20 min intervals to aid digestion.
- After 1 h add 10 ml of culture media to neutralize enzymatic activity and centrifuge the tube at 280 x g for 5 min at room temperature.
Note: It is at this stage that epithelial cells (HEECs) (see step C) or perivascular stem-cells (see step D) can be isolated for separate cultures. - After centrifugation, aspirate the supernatant, re-suspend the cell pellet in 15 ml of culture media and transfer to a 75 cm2 culture flask.
Note: Tilt the flask gently to distribute the cell suspension evenly across the culture surface. Avoid rotational mixing to risk concentrating cells in the center. - Incubate at 37 °C in a humidified 5% CO2 environment.
- Change the culture media after 6-18 h to remove blood cells, tissue debris and any unattached human endometrial epithelial cells (HEECs). Examination under the microscope should reveal sub-confluent stromal cells (Figure 2A).
Note: Confluency will vary depending on size and quality of biopsy. At this stage (see also Figure 6), immune cells can be collected from the supernatant and separated by antigen-specific magnetic separation or FACS, the details of which go beyond the remit of this protocol but readers are directed elsewhere (e.g., Manaster et al., 2008; Basu et al., 2009). - Cells will continue to proliferate (Figure 2B) until confluent (Figure 2C). Change the culture media every other day until passage (see step E) or assay.
Notes:- HEEC contamination (Figure 2D) should be minimal, please refer to ‘Notes’ section.
- Typical HESC yields vary considerably depending on digestion efficiency, mucus and blood content and varying cell attachment rates, but they will correlate with the size and quality of the tissue. As a guide we would expect to obtain 1-5 x 105 cells from a small biopsy (< 1 cm), 0.5-1 x 106 for an average biopsy (2-3 cm) and 1-2 x 106 for larger biopsies (> 4 cm) (counted 24 h after seeding). Readers are encouraged to allow cells to proliferate in culture until desired cell numbers are obtained.
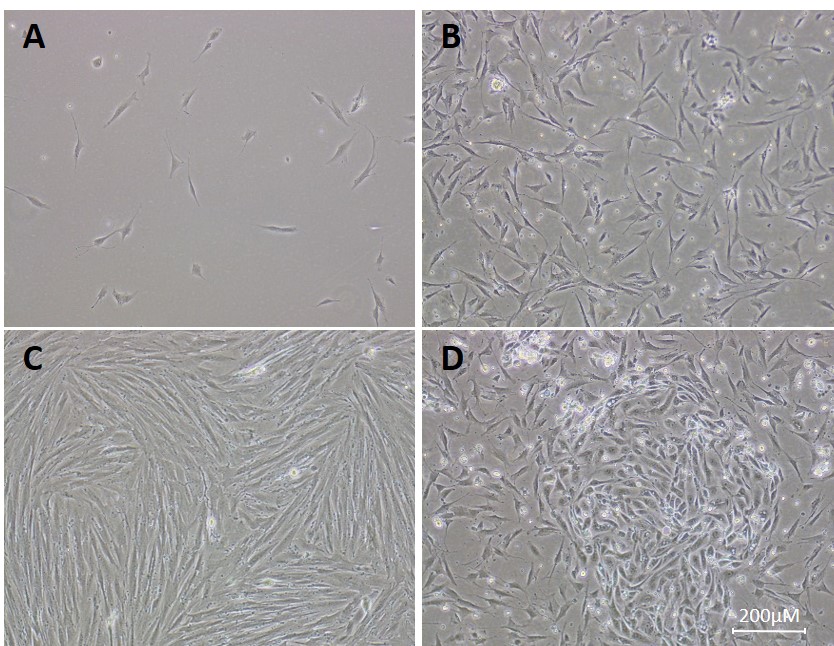
Figure 2. Cultured HESCs (bright-field microscope). A. HESC culture day one post-seeding. B. HESC culture day 3 post-seeding. C. Confluent culture of HESCs ready for passage or seeding. D. Culture of HESCs contaminated by HEECs, present as a whorl-like group of cells in the center.
- HEEC contamination (Figure 2D) should be minimal, please refer to ‘Notes’ section.
- Pre-prepare digestion media and pre-warm to 37 °C in a water bath.
- Separation of HEECs
- After digestion (step B8, refer also to Figure 6), filter the cell solution through a 40 µm nylon mesh cell-strainer. Flow-through will contain stromal, red blood and immune cells, but endometrial gland clumps are retained in the strainer (Figure 3A).
- Back-wash the filter using 20 ml of additive-free DMEM/F12 media and collect the glandular clumps in a 50 ml tube (Figure 3B).
- Centrifuge at 280 x g for 5 min, room temperature.
- Aspirate the supernatant and re-suspend the pellet in 1 ml 0.25% trypsin-EDTA to dissociate any clumps. Glandular clumps do not attach well to substrate and can be lost during media changes. This additional trypsin-dissociation step differs from previous protocols (Chen and Roan, 2015), and increases HEEC yield by dispersing clustered cells.
- Incubate the tube at 37 °C for 10 min.
- Add 9 ml of culture media and dissociate glandular clumps by vigorous pipetting up and down. A single cell solution of HEECs should result.
- Centrifuge at 280 x g for 5 min, room temperature.
- Aspirate the supernatant and re-suspend and seed the HEEC as required.
Notes:- HEEC yielding will depend on biopsy size and quality. Freshly isolated HEECs counted after step C8 typically yield 1-3 x 105 from small biopsies (< 1 cm), 3-9 x 105 for medium (2-3 cm) and 1 x 106 for large biopsies (> 4 cm). However, readers are encouraged to allow proliferation in culture to obtain desired numbers.
- The culture of HEECs requires non-standard culture techniques that go beyond the remit of this protocol. Readers are therefore directed elsewhere (e.g., Chan et al., 2004; Defrere et al., 2005; MacDonald et al., 2007).
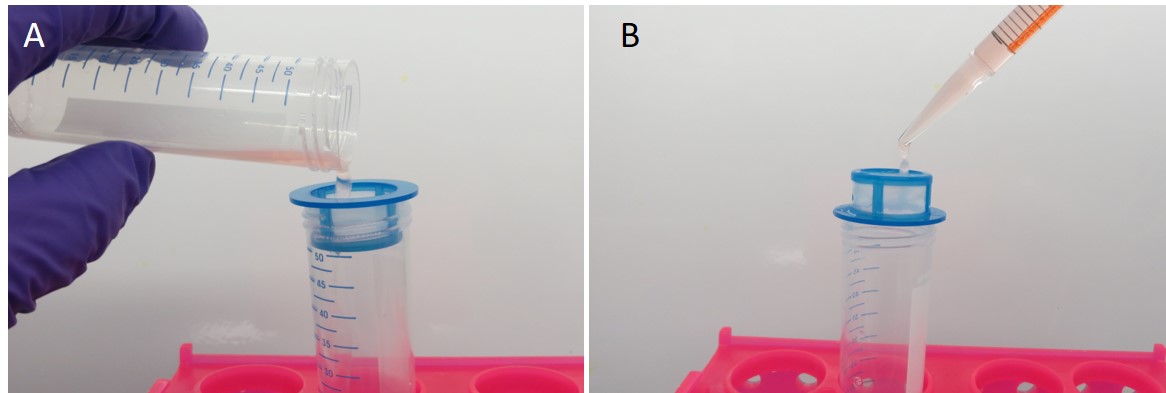
Figure 3. Separation and isolation of HESCs and HEECs. A. Following tissue digestion, collect HESCs as flow-through through a 40 μm cell strainer. B. Collect glandular epithelial clumps by back-washing (note inverted cell strainer) and disperse into single cell HEECs suspensions via trypsin incubation.
- HEEC yielding will depend on biopsy size and quality. Freshly isolated HEECs counted after step C8 typically yield 1-3 x 105 from small biopsies (< 1 cm), 3-9 x 105 for medium (2-3 cm) and 1 x 106 for large biopsies (> 4 cm). However, readers are encouraged to allow proliferation in culture to obtain desired numbers.
- After digestion (step B8, refer also to Figure 6), filter the cell solution through a 40 µm nylon mesh cell-strainer. Flow-through will contain stromal, red blood and immune cells, but endometrial gland clumps are retained in the strainer (Figure 3A).
- Isolation of endometrial perivascular progenitor mesenchymal stem-like cells
- Following step B8 (refer also to Figure 6), aspirate the supernatant and re-suspend the pellet in 8 ml culture media.
- Underlay 4 ml of Ficoll-paque to the bottom of the tube (Figure 4A). Ficoll-paque is a density gradient media used to separate out red blood cells.
Note: It is important to have two distinct layers before centrifugation (Figure 4B). To underlay Ficoll-paque, fill a 5 ml serological pipette, but dispense only 4 ml to the bottom of the tube, thus avoiding expulsion of bubbles and air mixing. Discard the remaining 1 ml. Dispense the 4 ml slowly and turn down the speed on the pipette-aid to its slowest setting. Avoid mixing at all times by handling carefully. Do not mix, knock or invert. - Centrifuge at 770 x g for 10 min, room temperature. Red blood cells will pellet and HESCs will reside within the interphase between the 2 layers (Figures 4C and 4D).
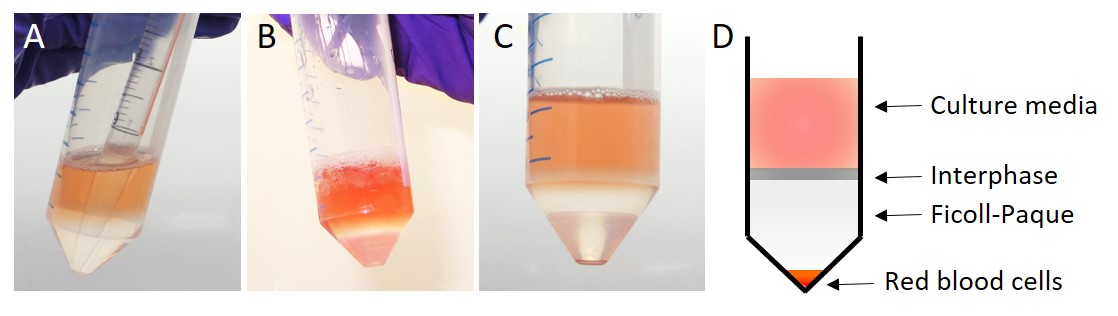
Figure 4. Separation of red blood cells using Ficoll-paque. A. Slowly underlay 4 ml Ficoll-paque beneath the digested tissue. B. 2 distinct layers should be visible. C-D. After centrifugation, red blood cells are pelleted and HESCs and HEECs as well as immune cells remain in the interphase from where they can be collected. - Carefully aspirate the majority of supernatant and collect the interphase containing the stromal cells and transfer to a 15 ml tube.
- Add 8 ml of culture media and mix well with a pipette.
- Wash cells free of Ficoll-paque by centrifugation (280 x g for 5 min, room temperature), aspiration of supernatant and resuspension in 10 ml culture media.
- Repeat step D6 twice more.
- Aspirate the supernatant and re-suspend the cell pellet in 5 ml of culture media and count the cells using an automated cell counter or haemocytometer.
- Centrifuge at 280 x g for 5 min, room temperature.
- Aspirate the supernatant and re-suspend the cell pellet in separation buffer (see Recipes) containing PE-conjugated W5C5 antibody. Use 100 µl of separation buffer and 5 µl of antibody per 106 cells.
- Mix well and incubate for 20 min in the dark at 4 °C.
- Wash the cells to remove unbound antibodies by adding 1 ml of separation buffer per 106 cells and centrifuge at 280 x g for 5 min at room temperature.
- Aspirate the supernatant completely and re-suspend the cell pellet in separation buffer containing anti-PE microbeads. Use 80 µl of buffer and 20 µl of anti-PE microbeads per 107 cells.
- Mix well and incubate for 20 min in the dark at 4 °C.
- Wash the cells by adding 1 ml of separation buffer per 106 cells and centrifuge at 280 x g for 5 min at room temperature.
- Aspirate the supernatant completely and re-suspend up to 107 cells in 500 µl of separation buffer.
- Place the MACS separator on the multi-stand and place the MS column in the MACS separator (Figure 5A).
- Mix the cell suspension by pipette, and apply to the column. Avoid adding air bubbles to the column.
- Collect unlabelled cells that pass through and wash MS column by addition of 500 µl separation buffer three times. Only add fresh 500 µl of buffer when the column reservoir is empty. Collect total effluent. This is the W5C5 negative fraction.
- Remove the MS column from the separator and place it in a sterile 15 ml tube.
- Immediately add 1 ml separation buffer into the column and flush out the magnetically labelled cells by firmly pushing the plunger into the column (Figure 5B). Flow-through will now contain the W5C5 positive fraction.
- To increase purity of the magnetically labelled cells, repeat magnetic separation on positive fraction (steps D18 to D21) using a new MS column.
- Centrifuge at 280 x g for 5 min at room temperature.
- Aspirate the supernatant, re-suspend the cell pellet and seed as required.
Although a high level of patient-to-patient variability is observed, isolated W5C5+ cells typically constitute between 4-8% of stromal cell populations (Murakami et al., 2013). They maintain many mesenchymal stem cell characteristics (Masuda et al., 2012), and can be cultured and differentiated (Ulrich et al., 2014) and assessed for clonogenicity using CFU (Colony Forming Units) assay (Masuda et al., 2012; Murakami et al., 2013; 2014) as required.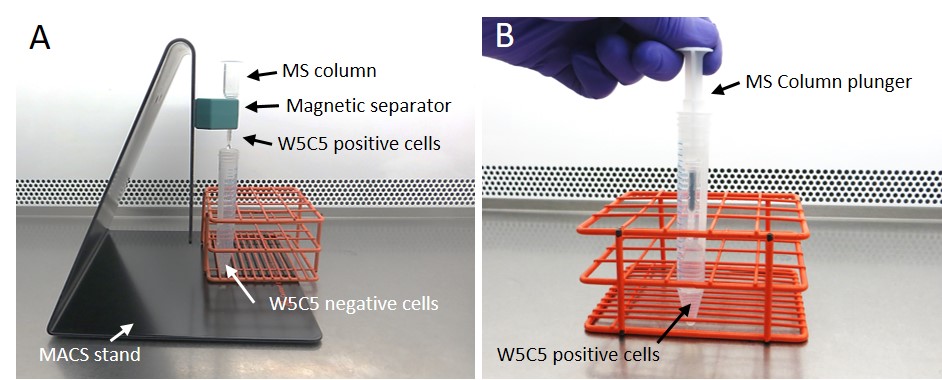
Figure 5. Magnetic separation of endometrial perivascular progenitor mesenchymal stem-like cells. Following antibody-labelling of W5C5+ cells (steps D9-D15), gravity-feed cell suspensions through columns in the magnetic stand. The flow-through will be the negatively labelled fraction (A). Remove the column from the magnetic stand and flush-through W5C5 positively labelled cells by immediate addition of 1 ml separation buffer and expulsion via the plunger (B). These steps can be repeated with the positive fraction using a fresh MS column to increase purity.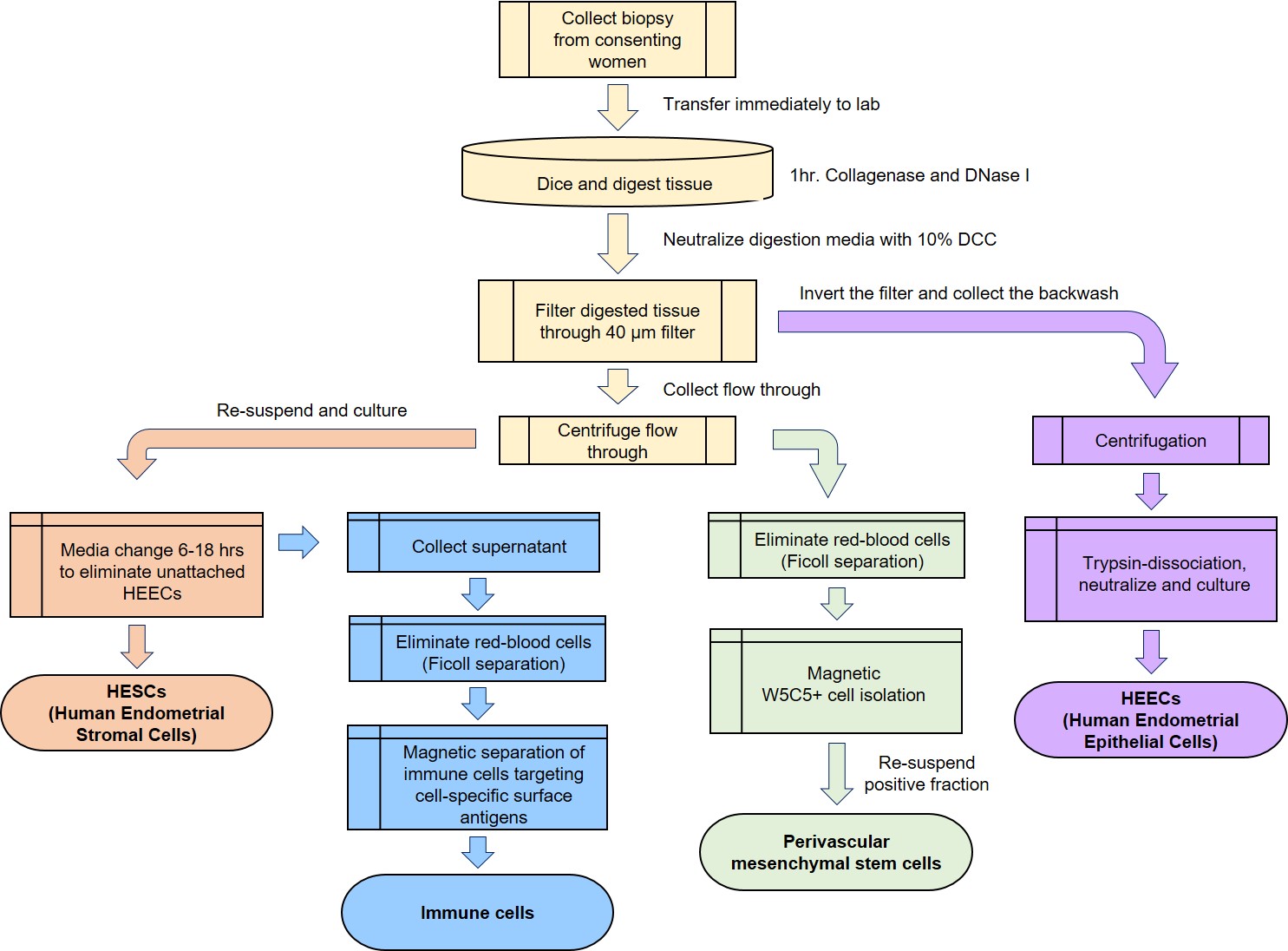
Figure 6. Work flow to separate different cell types within human endometrial biopsies
- Following step B8 (refer also to Figure 6), aspirate the supernatant and re-suspend the pellet in 8 ml culture media.
- Passage of HESCs
- Passage cells at 80-90% confluency (see Figure 2C).
- Pre-warm culture media, sterile PBS and 0.25% trypsin-EDTA to 37 °C in the water bath.
- Aspirate the culture media from the flask.
- Add 10 ml of PBS, rinse the cell monolayer and aspirate.
- Add 2 ml of 0.25% trypsin-EDTA solution, tilting the flask to ensure the solution covers the entire surface and return to incubator for 2-3 min or until cells have dislodged.
Note: Cells can be loosened by gentle agitation of the flask and checked under the light microscope. - Add 8 ml of culture medium into the flask to neutralize the trypsin and pipette the media repeatedly over the bottom of flask to wash any remaining cells.
- Transfer the cell suspension to a 15 ml tube and centrifuge for 5 min at 280 x g, room temperature.
- Re-suspend pellet in 9 ml culture media and seed 3 ml into a new 75 cm2 culture flask containing 12 ml culture media. For maintenance of culture, cells are usually split at a ratio of 1:3, but can at this stage be seeded into plasticware suitable for desired experiments.
- Passage cells at 80-90% confluency (see Figure 2C).
Notes
- When preparing DCC-FBS the vacuum filtration stops
- Filtration units are easily clogged. Avoid aspirating the charcoal from the bottom of the tube.
- Change the filtration system. Sometimes it takes two filter changes to accomplish total filtration.
- Filtration units are easily clogged. Avoid aspirating the charcoal from the bottom of the tube.
- Culture is contaminated with epithelial cells (see Figure 2D)
- After biopsy digestion, (step B8) filter the solution using a 40 µm cell strainer. The stromal cells pass through the filter and the majority of glandular clumps are retained.
- Perform the first media change earlier (3 to 6 h). Most stromal cells, but few epithelial cells, will be attached at this time.
- During the passage of the contaminated flask reduce the trypsin time to 2 min. Epithelial cells take longer to detach.
- After biopsy digestion, (step B8) filter the solution using a 40 µm cell strainer. The stromal cells pass through the filter and the majority of glandular clumps are retained.
- Few HESCs have attached and they are slow growing
- If the biopsy is too small, seed the cells in a 25 cm2 culture flask instead of 75 cm2.
- Check for infection. The presence of a bacterial or fungal infection would restrict cell growth by starving cells of nutrients. Infections manifest in different forms but readers should be concerned by cloudy media, fungal colonies, or bacterial or fungal spores viewed under a light microscope. If infection is suspected, discard culture and bleach cells and media to avoid repeat infections.
- If the biopsy is too small, seed the cells in a 25 cm2 culture flask instead of 75 cm2.
- The number of cells is too low even when the biopsy was large
- This may be due to inadequate digestion. Ensure tissue slicing/chopping removes all large pieces to aid digestion.
- Cell loss is possible during the Ficoll-paque separation. After the centrifugation with Ficoll-paque do not aspirate the top media phase. Transfer all media along with the interphase. Be sure to transfer the whole interphase even if you transfer a certain amount of Ficoll-paque along with it. The transferred Ficoll-paque will be cleared through washing.
- This may be due to inadequate digestion. Ensure tissue slicing/chopping removes all large pieces to aid digestion.
Recipes
Note: Ensure sterility. Work in a Class II microbiological safety cabinet and ensure full aseptic technique.
- Digestion media
10 ml phenol and additive-free DMEM/F12 culture media
0.5 mg/ml collagenase (prepare 50 mg/ml 100 µl stock aliquots)
0.1 mg/ml DNase I (prepare 10 mg/ml 100 µl stock aliquots) - Dextran-coated charcoal (DCC)-treated FBS
- Add 1.25 g of charcoal and 125 mg of dextran 70 to 500 ml of FBS, mix thoroughly and incubate at 56 °C in the water bath for 2 h, shaking every 30 min
- Transfer the FBS to 50 ml tubes and centrifuge at 1,800 x g, for 30 min
- Sterile-filter the supernatant using the vacuum-driven filtration system
- Aliquot to 50 ml volumes, label and store at -20 °C
- Add 1.25 g of charcoal and 125 mg of dextran 70 to 500 ml of FBS, mix thoroughly and incubate at 56 °C in the water bath for 2 h, shaking every 30 min
- Culture medium
500 ml DMEM/F-12 with phenol red
50 ml (10% [v/v]) DCC-FBS
5 ml (1% [v/v]) antibiotics/antimycotics
5 ml (1% [v/v]) L-glutamine
1 nM β-estradiol (prepare 100 µM stock in ethanol, store at -20 °C, add 5 µl)
2 μg/ml recombinant human insulin (prepare 10 mg/ml stock solution in acidified water [1.5%, v/v, acetic acid in sterile water, add 100 µl]) - Separation buffer (for magnetic separation of perivascular endometrial mesenchymal stem-like cells [0.5% BSA in PBS])
Dissolve 500 mg of BSA in 100 ml of sterile 1x PBS
Sterile-filter through 0.2 µm filter.
Store for up to a month at 4 °C before use
References
- Basu, S., Eriksson, M., Pioli, P. A., Conejo-Garcia, J., Mselle, T. F., Yamamoto, S., Wira, C. R. and Sentman, C. L. (2009). Human uterine NK cells interact with uterine macrophages via NKG2D upon stimulation with PAMPs. Am J Reprod Immunol 61(1): 52-61.
- Chan, R. W., Schwab, K. E. and Gargett, C. E. (2004). Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 70(6): 1738-1750.
- Chen, J. C. and Roan, N. R. (2015). Isolation and culture of human endometrial epithelial cells and stromal fibroblasts. Bio Protoc 5(20): e1623.
- Defrere, S., Van Langendonckt, A., Moulin, P., Befahy, P., Gonzalez, D., Martinez-Madrid, B., Dolmans, M. M. and Donnez, J. (2005). Human endometrial epithelial cells (EEC) constitutively express more intercellular adhesion molecule (ICAM)-1 than endometrial stromal cells (ESC) in culture. Am J Reprod Immunol 54(1): 5-12.
- MacDonald, E. M., Savoy, A., Gillgrass, A., Fernandez, S., Smieja, M., Rosenthal, K. L., Ashkar, A. A. and Kaushic, C. (2007). Susceptibility of human female primary genital epithelial cells to herpes simplex virus, type-2 and the effect of TLR3 ligand and sex hormones on infection. Biol Reprod 77(6): 1049-1059.
- Manaster, I., Mizrahi, S., Goldman-Wohl, D., Sela, H. Y., Stern-Ginossar, N., Lankry, D., Gruda, R., Hurwitz, A., Bdolah, Y., Haimov-Kochman, R., Yagel, S. and Mandelboim, O. (2008). Endometrial NK cells are special immature cells that await pregnancy. J Immunol 181(3): 1869-1876.
- Masuda, H., Anwar, S. S., Buhring, H. J., Rao, J. R. and Gargett, C. E. (2012). A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant 21(10): 2201-2214.
- Murakami, K., Bhandari, H., Lucas, E. S., Takeda, S., Gargett, C. E., Quenby, S., Brosens, J. J. and Tan, B. K. (2013). Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure--a pilot study. PLoS One 8(12): e82582.
- Murakami, K., Lee, Y. H., Lucas, E. S., Chan, Y. W., Durairaj, R. P., Takeda, S., Moore, J. D., Tan, B. K., Quenby, S., Chan, J. K., Gargett, C. E. and Brosens, J. J. (2014). Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology 155(11): 4542-4553.
- Ulrich, D., Tan, K. S., Deane, J., Schwab, K., Cheong, A., Rosamilia, A. and Gargett, C. E. (2014). Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum Reprod 29(9): 1895-1905.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Barros, F. S. V., Brosens, J. J. and Brighton, P. J. (2016). Isolation and Primary Culture of Various Cell Types from Whole Human Endometrial Biopsies. Bio-protocol 6(22): e2028. DOI: 10.21769/BioProtoc.2028.
Category
Stem Cell > Adult stem cell > Stromal cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









