- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Culture of Human Adipose-derived Stem Cells from Subcutaneous and Visceral White Adipose Tissue Compartments
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2027 Views: 16019
Reviewed by: Salma HasanAgnieszka PastulaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Human Adipose-derived Stem/Stromal Cells (ASCs) have been widely used in stem cell and obesity research, as well as clinical applications including cell-based therapies, tissue engineering and reconstruction. Compared with mesenchymal stem cells (MSCs) derived from other tissues such as umbilical cord and bone marrow, isolation of ASCs from human white adipose tissue (WAT) has great advantages due to its rich tissue source and simple surgical procedure. In this detailed protocol we describe a protocol to isolate and characterize ASCs from human WAT. Molecular characterization of isolated ASCs was performed through surface marker expression profiling using flow cytometry. Adipogenic capacity of the isolated ASCs was confirmed through inducing adipogenic differentiation and Oil Red O staining of lipid. This protocol provides researchers with the tools to culture and assess purity and adipogenic differentiation capacity of human ASCs, which can then be utilized for required downstream in vitro applications.
This protocol has been modified from Baglioni et al. (2009), Baglioni et al. (2012), and van Harmelen et al. (2005) to describe in detail a complete technique to isolate and subsequently characterize human ASCs from human WAT biopsies. This protocol has been utilized to isolate and characterize human ASCs from both subcutaneous and visceral WAT. The isolated human ASCs show high purity and demonstrate adipogenic differentiation capacity in vitro.
Background
Human ASCs are an invaluable in vitro cell model to study molecular pathways important for the etiology of metabolic diseases, including obesity and type 2 diabetes. Human ASC cultures derived from different WAT sources, including subcutaneous and visceral compartments, can also help us to understand functional differences between different WAT compartments. Short protocols have been published previously to describe the isolation of human ASCs from a maximum of two different WAT compartments (Baglioni et al., 2009; 2012; van Harmelen et al., 2005). Here we describe a detailed protocol for both, isolation and characterization of human ASCs from human WAT biopsies collected from several WAT compartments. This protocol has been used to reliably derive human ASCs from four different WAT compartments, including superficial subcutaneous, deep subcutaneous, omental and mesenteric WAT. The isolated primary cultures display homogeneous morphology and are pure, with a high percentage of cells displaying typical MSC marker expression. The isolated human ASCs also have the ability to differentiate into mature adipocytes, with accumulation of intracellular triglyceride droplets. In summary, this protocol reliably results in the isolation of pure human primary ASCs that maintain robust adipogenic differentiation capacity in vitro.
Materials and Reagents
- 15 ml centrifugation tube (Corning, Falcon®, catalog number: 352099 )
- 50 ml centrifugation tube (Corning, Falcon®, catalog number: 352070 )
- 10 cm cell culture dish (Greiner Bio One, CellStar®, catalog number: 664160 )
- 0.2 μm 25 mm syringe filter (Pall, Acrodisc®, catalog number: 4612 )
- 30 ml syringe (BD, Luer-LokTM, catalog number: 302832 )
- 5 ml polystyrene fluorescence activated cell sorting (FACS) tubes (Corning, Falcon®, catalog number: 352054 )
- 100 μm nylon mesh cell strainer (Corning, Falcon®, catalog number: 352360 )
- 6-well-plates (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140675 )
- Human WAT from patients
- OXOIDTM Phosphate buffered saline (PBS) tablets (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: BR0014G )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650 )
- Fetal bovine serum (FBS), heat inactivated (Thermo Fisher Scientific, GibcoTM, catalog number: 16140071 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906 )
- Antibodies (see Table 1)
- Isopropanol (EMD Millipore, catalog number: 109634 )
- Collagenase type IA (Sigma-Aldrich, catalog number: C9891-1G )
- Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: A0171 )
Note: This product has been discontinued. - Potassium bicarbonate (KHCO3) (Sigma-Aldrich, catalog number: P7682 )
Note: This product has been discontinued. - 0.25% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Dulbecco’s modified Eagle medium: nutrient mixture F-12 (DMEM/F-12) (1:1) (Thermo Fisher Scientific, GibcoTM, catalog number: 11330032 )
- Penicillin-streptomycin (P/S) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Dexamethasone (Sigma-Aldrich, catalog number: D4902 )
- 10 mg/ml insulin solution from bovine pancreas (Sigma-Aldrich, catalog number: I0516 )
- 3-isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, catalog number: I5879 )
- Indomethacin (Sigma-Aldrich, catalog number: 17378 )
- 100% ethanol (EMD Millipore, catalog number: 100983 )
Table 1. Antibody informationAntigen AntibodyManufacturerDilutionHost/IsotypeCD34 hematopoietic cells Anti-Human CD34 APC1:100Mouse IgG1, kappaCD31 Endothelial cells Anti-Human CD31 APC1:100Mouse IgG1, kappaCD14 (Macrophages) hematopoietic cells Anti-Human CD14 APC1:100Mouse IgG1, kappaCD11b (Leukocytes) Monocytes Anti-Human CD11b APC1:100Mouse IgG1, kappaCD45 (Nucleated cells of hematopoietic origin) lymphocytes Anti-Human CD45 APC1:100Mouse IgG1, kappaCD106 (Activated) endothelial cells Anti-Human CD106 PE1:100Mouse IgG1, kappaCD90 MSCs Anti-Human CD90 APC1:100Mouse IgG1, kappaCD44 MSCs Anti-Human CD44 APC1:100Rat IgG2b, kappaCD29 MSCs Anti-Human CD29 APC1:100Mouse IgG1, kappaCD73 MSCs Anti-Human CD73 APC1:100Mouse IgG1, kappaCD105 MSCs Anti-Human CD105 APC1:100Mouse IgG1 - Paraformaldehyde (Sigma-Aldrich, catalog number: P6148 )
- Oil Red O powder (Sigma-Aldrich, catalog number: O0625 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 221465 )
- Collagenase solution (1 mg/ml) (see Recipes)
- Red blood cell (RBC) lysis buffer (10x) (see Recipes)
- Proliferation medium (see Recipes)
- Differentiation medium (see Recipes)
- Induction medium (see Recipes)
- Insulin medium (see Recipes)
- 10 mM dexamethasone stock solution (see Recipes)
- 1 mM dexamethasone working solution (see Recipes)
- 0.5 M IBMX stock solution (1,000x) (see Recipes)
- 200 mM indomethacin stock solution (1,600x) (see Recipes)
- 4% paraformaldehyde (PFA) (pH = 7.4) (see Recipes)
- Oil Red O working solution (see Recipes)
Equipment
- Cryogenic vials (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 366656 )
- Laminar flow tissue culture hood
- Sterilized surgical tools including forceps and scalpel or scissors
- Mr. FrostyTM freezing container (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 5100-0001 )
- Locator 6 Plus Rack and Box Systems, liquid nitrogen Dewar (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: CY50985-70 )
- BD FACSCanto II flow cytometer (BD, model: BD FACSCanto II ) or similar equipment
- Analytical balance (Sartorius, model: CPA124S )
- MaxQTM 37 °C orbital shaker (Thermo Fisher Scientific, Thermo ScientificTM, model: 4450 )
- Centrifuge with swinging bucket rotor (KUBOTA, model: 2800 )
- Automated cell counter (Thermo Fisher Scientific, Countess®, catalog number: AMQAF1000 )
Software
- FACSDiva software (BD)
Procedure
- Cell isolation
- Collect 0.2-1.0 g of human WAT from patients
Note: We have utilized this protocol to isolate ASCs from superficial subcutaneous WAT isolated from morbidly obese individuals who underwent either gastrectomy or gastric bypass surgical procedures, as previously described (Leow et al., 2016). However, in unpublished studies from our lab we have also utilized this protocol to successfully isolate ASCs from deep subcutaneous, omental and mesenteric WAT from lean patients who underwent laparoscopic inguinal and ventral hernia surgery. - Transfer human WAT biopsy into pre-weighed 50 ml tube with PBS.
Note: A maximum of 1 g of WAT should be digested in one 50 ml centrifugation tube. - Calculate WAT tissue weight.
Note: For the following steps work in a laminar flow tissue culture hood.
- Transfer tissue into a 10 cm cell culture dish and mince the tissue into small pieces with sterilized scalpel or scissors.
Note: For better tissue digestion, WAT should be minced thoroughly. - Transfer the minced tissue into 50 ml tube. Add 3 ml collagenase solution/1 g of tissue. For tissue less than 0.3 g, use 1 ml of collagenase solution.
Note: Collagenase solution should be prepared freshly with pre-warmed PBS (from 37 °C water bath). - Digest the tissue in 37 °C incubator with shaking (120 rpm) for 1.5 h.
Note: No chunks of WAT should be visible after digestion. If chunks of WAT are still visible then digestion time can be extended accordingly. - Inactivate collagenase by adding 35 ml of proliferation medium into the 50 ml tube.
- Immediately centrifuge at 800 x g for 10 min at room temperature.
- After centrifugation the cell pellet should be visible at the bottom of the 50 ml tube.
- Carefully aspirate the supernatant (including fat layer) and re-suspend the cell pellet in RBC lysis buffer. For 1 ml of pellet, add 9 ml of RBC lysis buffer (1:9, v:v).
- Incubate for 10 min at room temperature.
- Immediately centrifuge at 800 x g for 10 min at room temperature.
- Re-suspend the cell pellet in 20 ml proliferation medium by pipetting up and down.
- Filter the cell suspension through a 100 μm nylon mesh cell strainer into a new 50 ml tube to remove debris.
- Immediately centrifuge at 800 x g for 10 min at room temperature.
- Carefully aspirate the supernatant and re-suspend the cell pellet containing ASCs in 10 ml proliferation medium.
- Transfer the cell suspension onto a cell culture treated 10 cm plate and incubate at 37 °C/5% CO2 for 24 h.
- After 24 h cell culturing, non-adherent cells are removed and adherent cells are washed with PBS. Adherent cells (ASCs) are cultured in fresh proliferation medium until 70% confluence.
- Passage ASCs 1-2 times to expand cell population. A representative image of growing ASCs is shown in Figure 1A. After several days of culture human ASCs show a typical fibroblastic-like morphology (Figure 1A).
- ASCs can then be either utilized for relevant in vitro investigations or cryopreserved. To cryopreserve ASCs, trypsinize and count 0.5-1 million cells and centrifuge at 800 x g for 10 min at room temperature. Aspirate the supernatant and re-suspend the cell pellet in 1 ml of pre-chilled (4 °C) proliferation medium containing 5% DMSO in cryogenic vials. Store vials in Mr. FrostyTM freezing container filled with isopropanol at -80 °C overnight, before transferring to a liquid nitrogen Dewar for long-term storage.
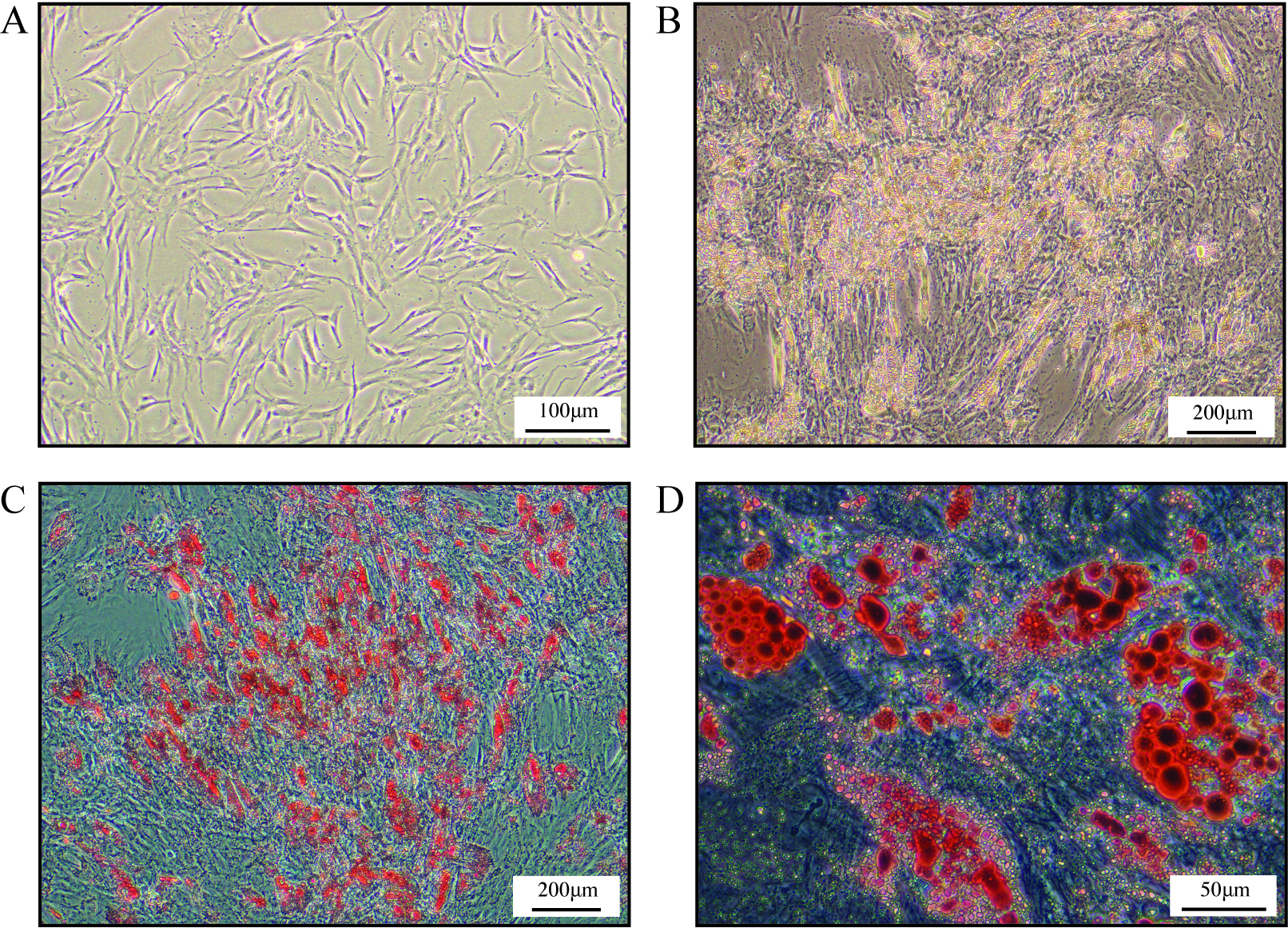
Figure 1. Human ASCs and ASCs-derived mature adipocytes. A. Representative phase contrast image of proliferating human ASCs cultures isolated from superficial subcutaneous WAT (Scale bar = 100 μm). B. Representative phase contrast image of human ASCs-derived mature adipocytes (Scale bar = 200 μm). C. Representative phase contrast image of Oil Red O stained lipid droplets within ASCs-derived mature adipocytes (Scale bar = 200 μm). D. Representative high magnification phase contrast image of Oil Red O stained lipid droplets within ASCs-derived mature adipocytes (Scale bar = 50 μm).
- Collect 0.2-1.0 g of human WAT from patients
- Cell surface markers staining and flow cytometry
- Trypsinize ASCs and count cell number.
- Collect ~0.2 million cells in each 5 ml FACS tube.
- Immediately centrifuge at 200 x g for 5 min at 4 °C and aspirate the supernatant.
- Wash the cell pellet with 3 ml PBS twice.
- Add 2 ml of ice-cold 4% PFA to each cell pellet and re-suspend the cell pellet by vortexing gently.
- Incubate at 4 °C for 30 min or keep the fixed cells at 4 °C for subsequent immunofluorescent staining (up to 6 months).
- Wash the fixed cells with 3 ml of PBS twice.
- Re-suspend the cell pellet with 1 ml of 15% FBS in PBS and incubate for 20 min for blocking.
- Immediately centrifuge at 200 x g for 5 min and aspirate the supernatant.
- Re-suspend the cell pellet with 50 μl of primary antibody diluted in ice-cold 15% FBS in PBS (For antibody information please see Table 1).
- Incubate on ice for 45 min.
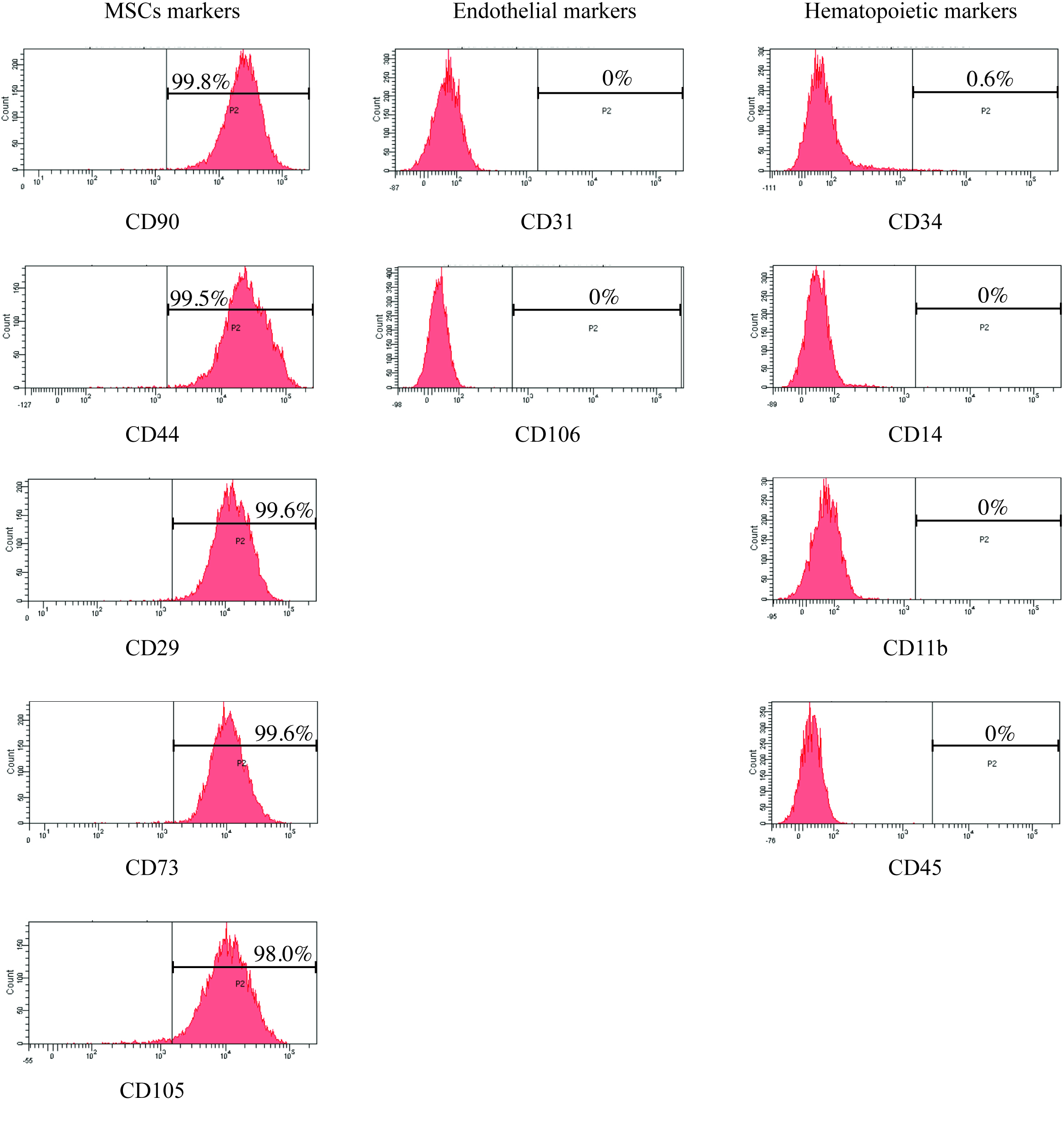
Figure 2. Surface marker expression profile analysis of human ASCs by flow cytometry. Isolated human ASCs (from superficial subcutaneous WAT) were analyzed for MSC markers (CD90, CD44, CD29, CD73 and CD105), hematopoietic lineage markers (CD34, CD14, CD11b, and CD45) and endothelial markers (CD31 and CD106) by flow cytometry. Histograms show results of staining of ASCs for indicated surface markers from one representative experiment. Cell counts are indicated on the y-axis and fluorescence intensity on the x-axis. Gate P2 was set according to staining with appropriate isotype-matched antibody controls. The percentages of ASCs stained positively are indicated in each panel. - Add 5 ml of ice-cold 0.5% BSA in PBS.
- Immediately centrifuge at 200 x g for 5 min at 4 °C and aspirate the supernatant.
- Re-suspend the cell pellet with 200 μl of ice-cold 0.5% BSA in PBS.
- Analyze samples using the BD FACSCanto II machine (BD) and FACSDiva software (BD) software for data acquisition.
Note: Alternative FACS hardware and software packages can be utilized. - Typically we find that more than 98% of the isolated ASCs express MSC markers, including CD90, CD44, CD29, CD73 and CD105. However, less than 1% of the isolated ASCs express cell surface markers for hematopoietic cells (CD34, CD14, CD11b, and CD45) and markers for endothelial cells (CD31 and CD106) (Figure 2).
- Trypsinize ASCs and count cell number.
- Adipogenic differentiation
- Trypsinize ASCs and count cell number.
- Plate cells on cell culture treated 6-well-plates in proliferation medium (see Recipes) at the density of 10,000 cells/cm2.
- Let cells grow to confluency and wait for another 48 h to arrest cell division.
- Treat cells with induction medium (Differentiation medium, containing 1 μM dexamethasone [diluted from 10 mM dexamethasone stock solution], 58 μg/ml insulin, 0.5 mM IBMX, and 200 μM indomethacin, see Recipes) for 2 weeks (We have observed that for longer term and more complex experimental studies [Leow et al., 2016], the treatment with induction medium can be decreased to 1 week). Change medium with fresh induction medium every 3 days. Treat cells with insulin medium (Differentiation medium, containing 10 μg/ml insulin, see Recipes). Change medium with fresh insulin medium every 2 days. Cells can be assayed from day-4 to day-7 post treatment with insulin medium. A representative image of differentiated ASC cultures is shown in Figure 1B, clusters of mature adipocytes filled with lipid droplets are visible.
- To assess lipid accumulation, Oil Red O staining is performed on differentiated mature adipocytes (Ramirez-Zacarias et al., 1992). For Oil Red O staining, remove medium and wash the cells with 2 ml of PBS/well. Fix the cells with 2 ml of 4% paraformaldehyde (PFA)/well for 30 min at room temperature before washing the cells with 4 ml of H2O/well. Incubate the cells with 2 ml of 60% isopropanol/well for 5 min. Aspirate the isopropanol and incubate the cells with 2 ml of Oil Red O working solution/well for 30 min at room temperature. Aspirate Oil Red O working solution and wash the cells with H2O before taking images. Representative images of Oil Red O stained mature adipocytes are shown in Figures 1C (4x objective) and 1D (20x objective). Oil Red O stained lipid droplets are clearly visible.
- Trypsinize ASCs and count cell number.
Data analysis
For characterization of surface marker expression of isolated human ASCs, flow cytometry data were collected with acquisition of 10,000 events per sample using the BD FACSCanto II machine (BD). Gate P2 was set according to staining intensity of appropriate isotype-matched control antibodies. Data analysis was performed using FACSDiva software (BD). Only representative data from one ASC culture are shown in this protocol. However, this protocol has been used to assess purity of several independent ASC cultures, with similar results obtained. No statistical analysis was performed in this protocol.
Notes
- This protocol has been used to isolate human ASCs from several WAT biopsies and the protocol has proved to be reproducible.
- The highest passage of ASCs we have used to study adipogenic differentiation potential is passage 10. However, a previous study has stated that ASCs maintain adipogenic differentiation capacity through multiple passages (up to at least passage 15) (Dicker et al., 2005).
Recipes
- Collagenase solution (1 mg/ml) (prepare freshly)
10 mg collagenase type IA
200 mg BSA
Top up with pre-warmed (37 °C) PBS to final volume of 10 ml
Filter sterilize using a 0.22 μm syringe filter before use - Red blood cell (RBC) lysis buffer (10x)
824 mg NH4Cl
100 mg KHCO3
4 mg EDTA
Top up to 100 ml with PBS
Filter sterilize using a 0.22 μm filter unit before use - Proliferation medium
DMEM/F-12
20% FBS
1% P/S
Filter sterilize using a 0.22 μm filter unit before use - Differentiation medium
DMEM/F-12
10% FBS
1% P/S
Filter sterilize using a 0.22 μm filter unit before use. - Induction medium (prepare freshly)
Differentiation medium
1 μM dexamethasone
58 μg/ml insulin
0.5 mM IBMX
200 μM indomethacin - Insulin medium (prepare freshly)
Differentiation medium
10 μg/ml insulin - 10 mM dexamethasone stock solution (2,000x)
Reconstitute 78.5 mg dexamethasone in 20 ml of 100% ethanol
Filter sterilize using a 0.22 μm syringe filter and store at 4 °C - 1 mM dexamethasone working solution
Dilute dexamethasone stock solution into 1 mM of dexamethasone working solution with PBS before using. - 0.5 M IBMX stock solution (1,000x)
Reconstitute 1 g of IBMX in 9 ml of DMSO
Filter sterilize using a 0.22 μm syringe filter
Aliquot into 1 ml fractions and store at -20 °C - 200 mM indomethacin stock solution (1,600x)
Reconstitute 71.567 mg of indomethacin in 1 ml of DMSO
Filter sterilize using a 0.22 μm syringe filter and store at -20 °C - 4% paraformaldehyde (PFA) (pH = 7.4)
Add 4 g of paraformaldehyde to 48 ml of H2O
Heat to dissolve
Add 2 N NaOH dropwise until solution becomes clear
Add 50 ml of 2x PBS and mix
Remove from heat and cool down the solution
Adjust pH to 7.4
Aliquot into 10 ml fractions and store at -20 °C - Oil Red O working solution (freshly prepared)
Dissolve 300 mg of Oil Red O powder into 100 ml of 99% isopropanol as Oil Red O stock solution (store at room temperature)
To prepare Oil Red O working solution, mix 3 parts of Oil Red O stock solution and 2 parts of H2O and incubate at room temperature for 10 min
Filter the Oil Red O working solution through filter paper
Use the filtered working solution for staining within 2 h
Acknowledgments
We would like to thank Khin Thida Soe for patient recruitment and coordinating human WAT biopsy collection at National University Hospital (NUH), Singapore. This work was supported by Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR) core funding. We also acknowledge the previously published protocols from Baglioni et al. (2009), Baglioni et al. (2012) and van Harmelen et al. (2005), based on which this current protocol was modified.
References
- Baglioni, S., Cantini, G., Poli, G., Francalanci, M., Squecco, R., Di Franco, A., Borgogni, E., Frontera, S., Nesi, G., Liotta, F., Lucchese, M., Perigli, G., Francini, F., Forti, G., Serio, M. and Luconi, M. (2012). Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One 7(5): e36569.
- Baglioni, S., Francalanci, M., Squecco, R., Lombardi, A., Cantini, G., Angeli, R., Gelmini, S., Guasti, D., Benvenuti, S., Annunziato, F., Bani, D., Liotta, F., Francini, F., Perigli, G., Serio, M. and Luconi, M. (2009). Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J 23(10): 3494-3505.
- Dicker, A., Le Blanc, K., Astrom, G., van Harmelen, V., Gotherstrom, C., Blomqvist, L., Arner, P. and Ryden, M. (2005). Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res 308(2): 283-290.
- Leow, S. C., Poschmann, J., Too, P. G., Yin, J., Joseph, R., McFarlane, C., Dogra, S., Shabbir, A., Ingham, P. W., Prabhakar, S., Leow, M. K., Lee, Y. S., Ng, K. L., Chong, Y. S., Gluckman, P. D. and Stunkel, W. (2016). The transcription factor SOX6 contributes to the developmental origins of obesity by promoting adipogenesis. Development 143(6): 950-961.
- Ramirez-Zacarias, J. L., Castro-Munozledo, F. and Kuri-Harcuch, W. (1992). Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 97(6): 493-497.
- van Harmelen, V., Skurk, T. and Hauner, H. (2005). Primary culture and differentiation of human adipocyte precursor cells. Methods Mol Med 107: 125-135.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ge, X., Leow, S. C., Sathiakumar, D., Stünkel, W., Shabbir, A., So, J. B. Y., Lomanto, D. and McFarlane, C. (2016). Isolation and Culture of Human Adipose-derived Stem Cells from Subcutaneous and Visceral White Adipose Tissue Compartments. Bio-protocol 6(22): e2027. DOI: 10.21769/BioProtoc.2027.
Category
Stem Cell > Adult stem cell > Stromal cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link