- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
An in vitro Model of Neuron-macrophage Interaction to Generate Macrophages with Neurite Outgrowth Properties
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2012 Views: 8972
Reviewed by: Soyun KimAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Time-Lapse Super-Resolution Imaging and Optical Manipulation of Growth Cones in Elongating Axons and Migrating Neurons
Masato Sawada [...] Kazunobu Sawamoto
Mar 20, 2025 2137 Views

Assay for Site-Specific Homologous Recombination Activity in Adherent Cells, Suspension Cells, and Tumor Tissues
Yuki Yoshino [...] Natsuko Chiba
Apr 5, 2025 2444 Views

Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Hanki Kim [...] Jun Young Choi
Jun 20, 2025 1425 Views
Abstract
Macrophages are known to play beneficial roles in axon regeneration after nerve injury. To develop an in vitro model in which injury signals can elicit pro-regenerative macrophage activation, we established co-cultures consisting of adult dorsal root ganglia sensory neurons and peritoneal macrophages and added cAMP analogue dibutyryl cAMP. The conditioned medium collected from the co-cultures exhibited robust neurite outgrowth activities. The neurite outgrowth activities were almost completely abrogated by addition of minocycline, a macrophage deactivator, indicating that factors responsible for neurite outgrowth are produced by activated macrophages.
Background
CNS neurons of adult mammals do not spontaneously regenerate axons after injury. Preconditioning peripheral nerve injury allows the dorsal root ganglia (DRG) sensory axons to regenerate central branches by promoting expression of regeneration-associated genes. We have previously showed that activated macrophages in the DRG following preconditioning injury critically contribute to enhancement intrinsic regeneration capacity of the DRG sensory neuron (Kwon et al., 2013). To identify molecular factors involved in the activation of macrophages following nerve injury, we have developed an in vitro model in which neuron-macrophages interactions are elicited by cAMP, a well-known reagent to enhance regenerative capacity of neurons. Compared to the previous model to activate macrophages using zymosan, our model utilizes a more physiologic stimulus resembling molecular events in the preconditioning peripheral injury model.
Materials and Reagents
- 1.5 ml Eppendorf tubes
- 15 ml conical tube
- Cell culture insert with 0.4 μm transparent PET membrane (Corning, Falcon®, catalog number: 353090 )
- 70 μm nylon cell strainer (Corning, Falcon®, catalog number: 352350 )
- 50 ml conical tube
- 6 well plate
- 0.2 μm filter (BD)
- 8-well culture slide (Corning, BiocoatTM, catalog number: 354632 )
- Dulbecco’s modified Eagle medium (DMEM) (GE Healthcare, HycloneTM, catalog number: SH30243.01 )
- Collagenase from Clostridium histolyticum (Sigma-Aldrich, catalog number: C9407-100MG )
- Neurobasal® medium (Thermo Fisher Scientific, GibcoTM, catalog number: 21103-049 )
- B-27® supplement (50x), serum free (Thermo Fisher Scientific, GibcoTM, catalog number: 17504-044 )
- GlutaMaxTM (Thermo Fisher Scientific, GibcoTM, catalog number: 35050-061 )
- Penicillin-streptomycin (10,000 U/ml, 10,000 µg/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122 )
- Poly-D-lysine hydrobromide (Sigma-Aldrich, catalog number: P6407-5MG )
- Laminin (Thermo Fisher Scientific, GibcoTM, catalog number: 23017-015 )
- Phosphate-buffered saline (PBS) (GE Healthcare, HycloneTM, catalog number: SH30256.01 )
- Red blood cell lysis buffer (QIAGEN, catalog number: 158904 )
- 10% FBS (GE Healthcare, HycloneTM, catalog number: SH30919.03 )
- Adenosine 3’,5’-cyclic monophosphate, N6,O2’-dibutyryl-, sodium salt (db-cAMP; 100 μM) (EMD Millipore, Calbiochem®, catalog number: 28745 )
- Minocycline (Sigma-Aldrich, catalog number: M9511 )
- Paraformaldehyde-13C (Sigma-Aldrich, catalog number: 604380 )
- Normal goat serum (Thermo Fisher Scientific, GibcoTM, catalog number: 16210072 )
- Triton X-100 (DAEJUNG CHEMICAL & MITALS, catalog number: 8566-4405 )
- Goat anti-mouse IgG (H + L) secondary antibody, Alexa Fluor® 594 conjugate (Thermo Fisher Scientific, InvitrogenTM, catalog number: A11005 )
- Anti β III tubulin (Tuj-1) (Promega, catalog number: G7121 )
- Neuron culture medium (see Recipes)
- Macrophage culture medium (see Recipes)
Equipment
- Fine scissors
- Fine forceps
- Hemacytometer (Marienfeld-Superior)
- Dissecting stereomicroscope (Carl Zeiss, model: Stemi DV4 )
- Cell culture CO2 incubator (Panasonic)
- Twist shaker (FINEPCR, model: Twist shaker Tw3t )
- Tabletop centrifuge (Sorvall)
- Pipette-aid
- Confocal microscope (Olympus, model: IX71 )
Software
- ImageJ (http://rsbweb.nih.gov/ij/index.html)
Procedure
- Adult DRG neurons are obtained from adult C57BL6 mice. After animals are sacrificed, skin and paravertebral muscles are dissected to expose the vertebral column. Bilateral dorsal root ganglia (DRGs) from the S1 up to the C1 level are freshly dissected using fine scissors and forceps under a dissecting microscope and soaked into fresh cold DMEM. Make sure to be careful not to damage DRGs when detaching the DRGs from the roots and nerves.
Note: It is critically important to dissect DRGs as quickly as possible. The collection of the total DRGs from one mouse should not take longer than 30 min.
- Transfer DRGs to a 1.5 ml Eppendorf tube with a blue pipette tip with an end cut off. Quickly spin down DRGs and aspirate off DMEM. Add 1 ml of DMEM containing 125 U/ml type XI collagenase and incubate for 90 min at 37 °C with gentle rotation using a twist shaker kept in an incubator. The optimal range for rotation speed is around 35-45 rpm.
- Aspirate off collagenase-containing DMEM and resuspend DRGs with 1 ml of DMEM. When DMEM is added, DRGs will float. Wait until DRGs fall down, and then aspirate off. Repeat this step at least five times to remove collagenase completely.
Note: Try not to aspirate off completely. It is important to prevent DRGs from being sucked out.
- Transfer DRGs to 15 ml conical tube using a cut off blue pipette tip. Then triturate at least 15 times using a blue tip until a homogenous suspension of cells is achieved.
Note: Trituration should be very gentle. Avoid making bubbles and try not to touch the bottom of conical tube.
- Centrifuge the tube at about 239 x g for 3 min to remove cell debris in supernatant. Resuspend cell pellets in 1 ml Neurobasal medium supplemented with B27 and triturate 5 to 10 times. Then pass the cells through a 70 μm cell strainer installed on a 50 ml conical tube. Wait for 2 min, and then pour Neurobasal/B-27 medium using a pipette-aid to the strainer so that cells passing the strainer come off easily. Wait one more minute.
- Plate dissociated cells onto a 6 well plate pre-coated with 0.01% poly-D-lysine and laminin (3 μg/ml), and shake the plate very gently for the cells to be evenly distributed. A total of 6 x 106 DRG neurons are plated for each well. Then, the plate is placed in a CO2 incubator maintained at 37 °C.
Note: Poly-D-lysine (0.01%) and laminin (3 μg/ml) are diluted with distilled water. Poly-D-lysine coats the plate for 2 h at 37 °C or overnight at 4 °C, after which the plate is washed twice with distilled water. Then, the plate is coated with laminin for 2 h at room temperature, and washed twice with PBS. Then the plate is dried at room temperature before cell plating.
- The co-cultures need to be set up 4 h after the initial plating of dissociated DRG neurons. Primary peritoneal macrophages are prepared from adult C57BL6 mice. After sacrificing mice in CO2 chamber, abdominal skin is delicately dissected to expose the peritoneum.
- Inject 10 ml of ice-cold PBS into the peritoneum. Gently massage the peritoneum for 1-2 min. Then, suck out PBS and transfer to a new 50 ml conical tube.
- The lavage fluid is centrifuged at 239 x g for 10 min at 4 °C to pellet the cells. The cell pellets are resuspended in 3 ml of the red blood cell lysis buffer for 3 min at room temperature (RT) and then centrifuged at 239 x g for 10 min at 4 °C to pellet the cells again.
- Pelleted cells are resuspended in macrophage culture medium. The peritoneal macrophages are plated on a cell culture insert placed on the 6 well plate where dissociated DRG neurons have been plated 4 h before. The number of plated macrophages is kept 5 times higher than that of cultured DRG neurons.
- 4 h after macrophage plating, the neuron–macrophage co-cultures are treated with dibutyryl-cAMP (db-cAMP; 100 μM) or PBS as a control. In some wells, macrophage deactivator minocycline is added with db-cAMP at a concentration of 10 μg/ml.
- After 24 h, the culture medium is replaced with fresh macrophage culture medium. The co-cultures are maintained for 72 h without changing the medium.
- Then the conditioned medium is collected, centrifuged at 239 x g for 5 min, and passed through a 0.2 μm filter to remove any remaining cellular debris. The collected conditioned medium is stored at -70 °C until use.
- For neurite outgrowth assays, adult DRG neurons are obtained and dissociated using the same method described in steps 1-3.
- Cell pellets are resuspended in Neurobasal-A supplemented with B27 and a total of 1 x 104 cells (per well) are plated onto eight-well culture slides pre-coated with 0.01% poly-D-lysine and 3 μg/ml laminin. Precoating is done using the same method described in step 6.
- The neuron cultures are maintained in an incubator for 2 h allowing for cell attachment to the bottom. And the culture medium is replaced with the thawed conditioned medium collected in step 15.
- After 15 h from the initial plating, the cells are directly fixed with 4% cold paraformaldehyde for 20 min.
- The fixed cells on slides are washed three times with 1x PBS and incubated with a blocking solution consisting of 10% NGS and 0.1% Triton-X for 30 min. Then, wash with 1x PBS twice, perform primary antibody (Tuj-1) staining at 1:1,000 dilution with 10% NGS for 4 h at RT or overnight at 4 °C.
- Wash twice with 1x PBS, and perform secondary antibody (goat-anti rabbit) staining at 1:500 dilution with 10% NGS for 2 h at RT. Then, wash the slides twice with 1x PBS and mount the coverslips on the slides with mounting gel.
- Take images using confocal microscope for immunostained β III tubulin to visualize neurite outgrowth.
Data analysis
- The mean neurite length per neuron is measured to compare the extent of neurite outgrowth induced by different conditioned medium. Neurite length is measured using the Neuron J plugin for the image analysis software suite NIH ImageJ (publicly available from http://rsbweb.nih.gov/ij/index.html), which facilitates tracing and quantification of elongated structures. Each experimental condition is replicated in two wells per culture. Each well is divided into four quadrants, and a 200x magnification image is obtained at the center of each quadrant (four images in each well). Neurites are traced for all neurons in each image, and the number of DRG neurons per image is counted manually. Approximately 20 neurons per image are observed, and, therefore, around 80 neurons are measured for each well. The average neurite length per neuron in each well is calculated by dividing the total neurite length from the total number of neurons.
- Conditioned medium collected from the neuron-macrophage co-cultures treated with PBS do not support neurite outgrowth (Figure 1A). When DRG neurons are grown with conditioned medium treated with dibutyryl cAMP, they invariable show robust neurite outgrowth (Figure 1B). If minocycline, a macrophage deactivator, is added to the neuron-macrophage co-cultures along with cAMP, neurite outgrowth activity is almost completely abolished, suggesting that the neurite outgrowth activity required activation of macrophage (Figure 1C). In our previous study, we showed that conditioned medium collected from either neuron or macrophage alone cultures with cAMP treatment did not support neurite outgrowth (Kwon et al., 2013). Therefore, the interaction between neurons and macrophages are critical for the neurite outgrowth activity.
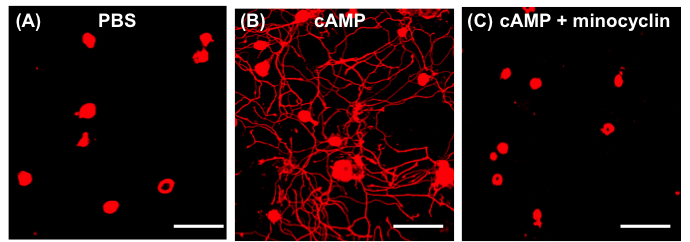
Figure 1. Neuron-macrophage interactions promote neurite outgrowth activity. Neuron (adult DRG neurons) and peritoneal macrophage co-cultures were treated with PBS (A), cAMP (B) and cAMP + minocycline (C). Scale bars = 100 μm.
Notes
- In the neurite outgrowth assay using this protocol, DRG neurons do not grow any significant neurites within 15 h in culture. However, if DRG neurons are allowed to grow longer than 15 h, some degree of neurite outgrowth can be observed even in control condition. Similar findings were reported in the neurite outgrowth assay using rat DRG neurons in the previous study (Cafferty et al., 2004). In pilot experiments, we test several different culture durations, and we found that 15 h of culture resulted in minimal neurite outgrowth in control condition while highly robust neurite outgrowth was achieved with conditioned medium treated with cAMP.
- To set up the neuron-macrophage co-cultures, dissociated macrophages can be added directly to cultured DRG neurons without cell culture insert. In our previous study, we directly compared the extent of neurite outgrowth between conditioned medium collected from direct co-cultures and from the co-cultures with the two cell types separated by cell culture insert (Kwon et al., 2013), and there was no difference between the two conditions. This suggests that the two cell types are communicating with each other using soluble molecules. The following study in our lab discovered that CCL2, secreted from neurons, is responsible to activate macrophages into a pro-regenerative phenotype in this co-culture model (Kwon et al., 2015).
Recipes
- Neuron culture medium
Neurobasal medium (500 ml)
2% B27
1% glutamax
1% penicillin-streptomycin
- Macrophage culture medium
DMEM
10% FBS
1% penicillin-streptomycin
Acknowledgments
This protocol is supported by a grant NRF-2015R1A2A1A01003410 from the Ministry of Science, ICT and Future Planning, Republic of Korea.
References
- Cafferty, W. B., Gardiner, N. J., Das, P., Qiu, J., McMahon, S. B. and Thompson, S. W. (2004). Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci 24(18): 4432-4443.
- Kwon, M. J., Kim, J., Shin, H., Jeong, S. R., Kang, Y. M., Choi, J. Y., Hwang, D. H. and Kim, B. G. (2013). Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci 33(38): 15095-15108.
- Kwon, M. J., Shin, H. Y., Cui, Y., Kim, H., Thi, A. H., Choi, J. Y., Kim, E. Y., Hwang, D. H. and Kim, B. G. (2015). CCL2 mediumtes neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J Neurosci 35(48): 15934-15947.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yun, H. J. and Kim, B. S. (2016). An in vitro Model of Neuron-macrophage Interaction to Generate Macrophages with Neurite Outgrowth Properties. Bio-protocol 6(22): e2012. DOI: 10.21769/BioProtoc.2012.
- Kwon, M. J., Shin, H. Y., Cui, Y., Kim, H., Thi, A. H., Choi, J. Y., Kim, E. Y., Hwang, D. H. and Kim, B. G. (2015). CCL2 mediumtes neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J Neurosci 35(48): 15934-15947.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Cell Biology > Cell isolation and culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link