- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Establishment of Patient-Derived Xenografts in Mice
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2008 Views: 15570
Reviewed by: Clara Lubeseder-MartellatoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Murine Orthotopic Allograft to Model Prostate Cancer Growth and Metastasis
Robert M. Hughes [...] Paula J. Hurley
Feb 20, 2017 12188 Views

Qualitative in vivo Bioluminescence Imaging
Devbarna Sinha [...] Pritinder Kaur
Sep 20, 2018 10949 Views
Abstract
Patient-derived xenograft (PDX) models for cancer research have recently attracted considerable attention in both the academy and industry (Hidalgo et al., 2014; Wilding and Bodmer, 2014). PDX models have been developed from different tumor types including lung cancer to improve the drug development process. These models are used for pre-clinical drug evaluation and can be used for the predictive results of clinical outcomes because they conserve original tumor characteristics such as heterogeneity, complexity and molecular diversity (Kopetz et al., 2012). Additionally, PDX model provides the potential tool for the personalized drug therapy. In this protocol, we present methods for the establishment of PDX in mice using primary tumor tissues from patients with small cell lung cancer (SCLC).
Materials and Reagents
- Sterile alcohol prep pads (Covidien, catalog number: 6818 )
- Petri dishes (Corning, Falcon®, catalog number: 353003 )
- Tissue adhesive (3M, catalog number: 1469SB )
- 6 weeks old SCID mice (Charles River Laboratories International, catalog number: 236 ) or athymic nude mice (Envigo, catalog number: 069 )
- Ketamine hydrochloride/xylazine hydrochloride solution (Sigma-Aldrich, catalog number: K113 )
- Phosphate-buffered saline (PBS) (Mediatech, catalog number: 21-040-CV )
- 70% ethanol (Decon Labs, catalog number: 2401 )
Equipment
- Stainless steel sterile scalpels (Integra LifeSciences, Miltex®, catalog number: 4-423 )
- Operating scissors (Sklar Surgical Instruments, catalog number: 13-1045 )
- Micro dissecting forceps (Roboz Surgical Instrument, catalog number: RS-5135 )
- Biological safety cabinet (Labconco, catalog number: 3460001 )
- CO2 chamber in animal facility
- Heating pad (Sunbeam Products, catalog number: 000765-500-000 )
Procedure
- Collect pieces of primary tumors by surgery or biopsy procedures (F0).
- Anesthetize the SCID mice using ketamine hydrochloride/xylazine hydrochloride solution.
- Remove hair from the dorsal region.
- Cut the tumor (F0) into pieces.
- Make a shallow incision in the dorsal region of SCID mouse using a scissor and then implant subcutaneously two pieces of primary tumor into the interscapular fat pad of SCID mouse within 3 h after collecting primary tumor tissues from a patient. These mice in the engraftment phase are called F1 mice (SCID mice are used for F1 mice due to higher engraftment rates than those of athymic nude mice).
- Move the F1 mice to a warm area on heating pad and monitor them during recovery.
- Return F1 mice to the routine mouse sterile housing.
- When the tumor grows and reaches around 1.5 cm (4-10 weeks), prepare the additional transplant.
- Place the F1 mouse in the CO2 chamber for 5 min to euthanize it.
- Move the euthanized mouse into biological safety cabinet and clean the whole body of mouse using sterile alcohol prep pads.
- Cut off skin around the tumor area.
- Resect the tumor and transfer the tumor to a sterile Petri dish containing PBS (Video 1).
- Cut the tumor into even size piece (i.e., 2 mm3) (Video 1).Video 1. The cutting of tumor into small even piece for engraftment

Figure 1. Establishment of PDX model. A. Photographs of representative small cell lung cancer (SCLC) PDX in mice (F3 mice). B. H&E histology of tumor tissues from SCLC PDX in mice. TKO stands for Dr. Taofeek K. Owonikoko who provided primary lung tumor tissues. - Anesthetize the athymic nude mice like described in step 2.
- Implant a piece of tumor into each experimental athymic nude mouse like described in step 5. Apply tissue adhesive to cut surfaces of the skin. Mice in this expansion phase are called F2 mice. After 2-4 weeks, the tumors could be visible as in Figure 1 and Video 2.
- Move the F2 mice to a warm area and monitor them like described step 5.
- Return the F2 mice to the routine mouse sterile housing.
- When the tumor volume reaches 100-150 mm3, mice can be used for downstream application.Video 2. The tumor implantation in mice
Notes
- Ensure that all procedures should be undertaken in a biological safety cabinet.
- Ensure that any necrotic tumor tissue is not used for transplant (Figure 2).
- Ensure that only early-passage PDXs (less than the 5th passage) can be used for the studies.
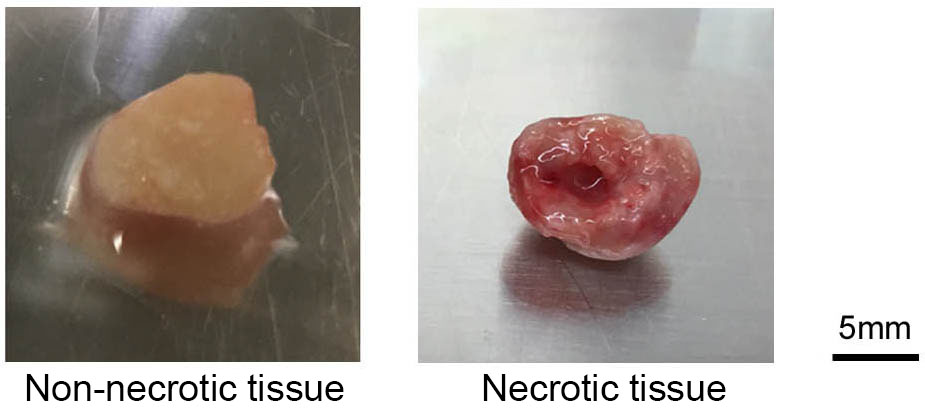
Figure 2. Photographs of representative non-necrotic tissue and necrotic tissue
Acknowledgments
We thank Dr. Taofeek K. Owonikoko, Assistant Professor and Guojing Zhang, Research Specialist at Emory University for providing primary lung tumor tissues. This work is supported by NCI, National Institutes of Health grants 1R01CA193828, 2R01CA136534 and 1R01CA200905-01A1.
References
- Hidalgo, M., Amant, F., Biankin, A. V., Budinska, E., Byrne, A. T., Caldas, C., Clarke, R. B., de Jong, S., Jonkers, J., Maelandsmo, G. M., Roman-Roman, S., Seoane, J., Trusolino, L. and Villanueva, A. (2014). Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 4(9): 998-1013.
- Kopetz, S., Lemos, R. and Powis, G. (2012). The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res 18(19): 5160-5162.
- Wilding, J. L. and Bodmer, W. F. (2014). Cancer cell lines for drug discovery and development. Cancer Res 74(9): 2377-2384.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Park, D., Wang, D., Chen, G. and Deng, X. (2016). Establishment of Patient-Derived Xenografts in Mice. Bio-protocol 6(22): e2008. DOI: 10.21769/BioProtoc.2008.
Category
Cancer Biology > Invasion & metastasis > Animal models > Cell invasion
Cancer Biology > General technique > Tumor formation
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link