- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Arabidopsis Seed Germination Assay with Gibberellic Acid
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2005 Views: 19851
Reviewed by: Arsalan DaudiYuan ChenAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Ethylene Production in Leaf and Bud Tissue of the Subtropical Tree Crop Litchi (Litchi chinensis Sonn.) Using Gas Chromatography and Flame Ionization Detection
Regina B. Cronje and Arnoldus J. Jonker
Mar 20, 2023 1555 Views

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1954 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views
Abstract
This assay analyzes Arabidopsis seed germination in response to gibberellic acid (GA). During seed imbibition, visible physiological changes allow precise determination of germination rate. This protocol utilizes a stereoscopic microscope to improve characterization of seed germination process.
Background
Seed germination is a critical process of the plant life cycle controlled by phytohormones, such as GA and abscisic acid (ABA), and environmental factors. Seed germination comprises two physiological processes, including seed coat (testa) and endosperm ruptures. Usually, penetration of endosperm by the radicle indicates that germination is complete. Previous studies generally use endosperm rupture to calculate germination rate. However, seed coat rupture also measures the progression of seed germination. This protocol utilizes a stereoscopic microscope to provide a visible and precise calculation of seed germination, including the rates of both seed coat and endosperm rupture (Zhong et al., 2015).
Materials and Reagents
- 1.5 ml Eppendorf tubes (Eppendorf)
- 950 x 150 mm Petri dish (Shanghai Wuyi Glass Factory, catalog number: 1771 )
- Sterile pipette tips (Corning, Axygen®)
- Parafilm (Bemis, catalog number: PM-996 )
- Aluminum foil (GLAD, catalog number: F5M )
- 0.2 µm syringe filters (Pall, Acrodisc®, catalog number: 4554 )
- Arabidopsis seeds
- Murashige and Skoog basal medium (Sigma-Aldrich, catalog number: M5519 )
- Agar (MBCHEM, catalog number: 170837 , agented by WHIGA in China)
- Sucrose, purity: AR (Tianjin Damao Chemical Reagent Factory, catalog number: 57-50-1 )
- Gibberellic acid (GA3) (Sigma-Aldrich, catalog number: G7645 )
- Ethanol, purity: AR (Tianjin Damao Chemical Reagent Factory, catalog number: 64-17-5 )
- Potassium hydroxide (KOH), purity: AR (Chengdu Institute of Chemical Reagents, catalog number: 1310-73-2 )
- MS solid medium (see Recipes)
- 10 mM GA3 stock (see Recipes)
- 70% ethanol (see Recipes)
- 1% sodium hypochlorite (see Recipes)
- Autoclaved distilled water (see Recipes)
Equipment
- Aquapro water purification system (Aquapro, model: AQ06062001 )
Note: This product has been discontinued. - Refrigerator (Haier, model: BCD-648WDBE )
- Autoclave (HIRAYAMA, model: HVE-50 )
- 1 ml pipette (Eppendorf, catalog number: 3120620.001 )
- Drying oven (ZenithLabo, model: DHG 9070A )
- 100 ml flasks
- Microwave oven (Galanz, model: G70F20N2L-DG )
- Benchtop (Suzhou Antai Air-tech, model: SW-CJ-1FD )
- Stereoscopic microscope (Nikon, model: SMZ1500 )
- Digital camera (10 megapixels) (Nikon, model: COOLPIX4500 )
- Thermometer (WUqiang, China)
Software
- Photoshop CS5 software
- IBM SPSS Statistics 22 software
- Origin8.0 software or Microsoft Excel
Procedure
- Preparation: All the reagents used in this protocol should be sterile (see Recipes for detailed information). Sterilize 1.5 ml Eppendorf tubes, Petri dishes, and 1 ml pipette tips by autoclaving (121 °C, 20 min). Dry sterilized materials overnight in a drying oven at 37 °C.
- Prepare the solid MS medium plates with or without GA3: Thoroughly melt solid MS medium (two flasks with 100 ml each) using a microwave oven and then cool flasks down to 50-60 °C. In one flask make a 1 µM GA3 solution (stock solution of 10 mM GA3), thoroughly but gently mix media, and then pour into four plates. Without adding GA3 pour the other flask of medium into another four plates. After cooling, seal all the plates with Parafilm and store at 4 °C before use.
Notes: - ½ MS medium is optional in this step.
- The medium must be cooled before adding GA3 stock solution.
- The medium can be prepared and kept at 4 °C before conducting experiments. However, the medium with GA3 should be kept in the dark and used within a week.
- The concentration of GA3 can be adjusted according to experimental needs.
- Collect approximately 200 mature seeds into a 1.5 ml Eppendorf tube.
- Sterilize seeds at the clean bench: Add 500 μl 70% (v/v) ethanol to the tube containing the seeds and vigorously invert it several times. After 3 min, discard the ethanol, add 1 ml 1% (v/v) sodium hypochlorite, and invert several times over the course of 10 min. Finally, rinse seeds four or five times with sterile distilled water.
Note: Make sure the time for sterilizing seeds is no longer than 3 min with ethanol and 10 min with sodium hypochlorite. - Sowing seeds: Resuspend seeds in approximately 100 μl of water and then transfer to media by using a pipette. When transferring, make sure seeds are spaced apart and not touching each other. At least 80 seeds should be sowed for each treatment. Leave plates open long enough so excess water around seeds and on media surface can evaporate. Wrap plates in aluminum foil to maintain darkness.
- Stratification: Keep all plates together at 4 °C for 2 or 3 days.
Note: Ensure the temperature of stratification to be 4 °C. Higher temperature will accelerate seed germination before the exposure to light. - After stratification, put the medium plates into a culture room (22 °C with a 16 h light/8 h dark photoperiod, the light supplied by cool and warm white fluorescent bulbs, a light intensity of approximately 100 μmol m-2 s-1 on the shelf surface, and 75%-80% relative humidity).
Notes: - Make sure that the culture temperature is 22 °C, and a thermometer should be kept on the shelf surface. Higher temperature will affect seed germination.
- Make sure that all media plates are applied with the same intensity of light. Due to the variation of light intensity of single bulbs, plates should be kept in a narrow scope.
- Data recording: Mark 0 h when seeds are exposed to light. Using a stereoscopic microscope (5x magnification) take photos of each plate every 2 h until all seeds have germinated (see Figure 1). Data should be calculated based on at least three independent experiments with similar results.
Note: It is optional to count seed germination after the radical tip emergence (see Figure 1, right panel), which is defined as the first sign of seed germination. Seeds are scored every 2 h until seed germination rate reaches over 98%.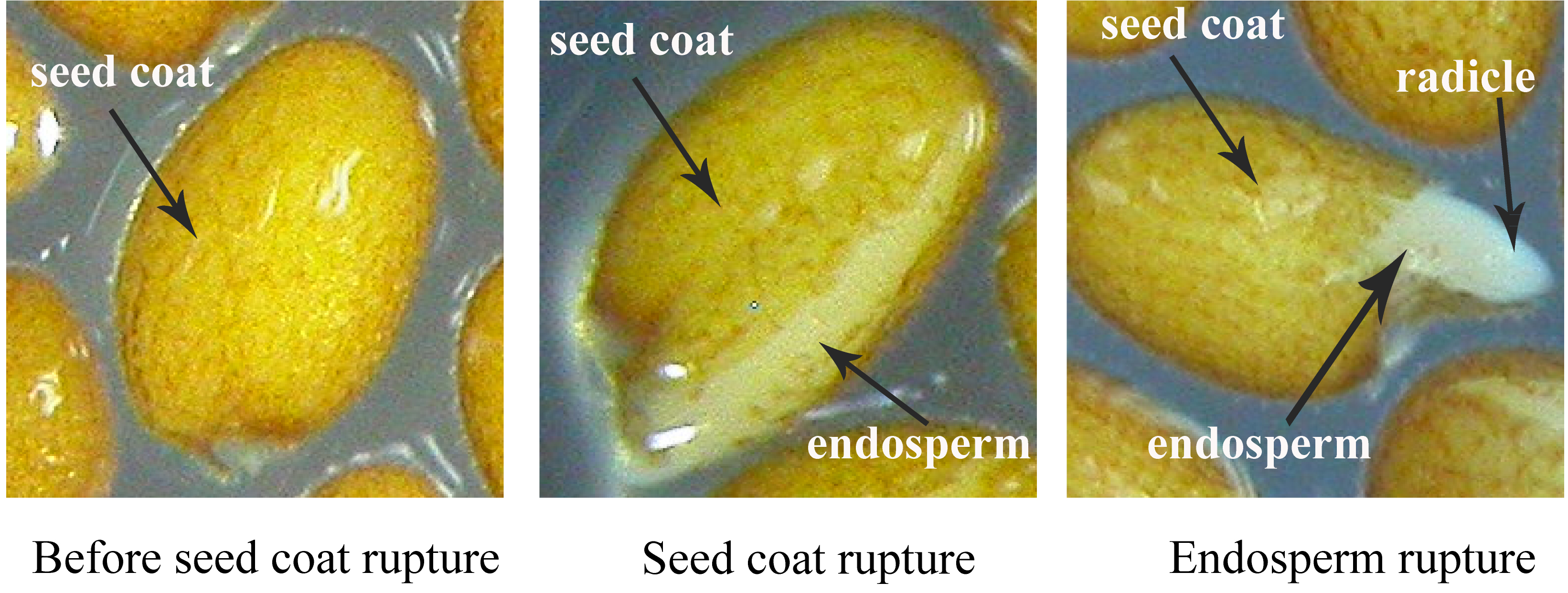
Figure 1. Seed germination process of Arabidopsis thaliana. Images show the status of seeds during seed coat and endosperm rupture under the normal condition. Photographs were taken before (left) or after seed coat rupture (middle) or after endosperm rupture (right). Seed coat, endosperm, and radicle are indicated by black arrows.
Data analysis
Using Photoshop CS5 software, count the number of the seeds with a ruptured testa or endosperm at each time point. The germination rate is indicated by the percentage of seeds with a ruptured endosperm compared to the total number of seeds. Additionally, the rate of testa rupture is indicated by the percentage of seeds with a ruptured testa compared to the total number of seeds (optional). Analyze the germination and testa rupture rates by using the IBM SPSS Statistics 22 software. Significant differences in comparison with control seeds (*P < 0.05, **P < 0.01), are analyzed by one-way analysis of variance (ANOVA). The line diagram of seed germination is made by using Origin8.0 software or Microsoft Excel.
Notes
- From the step of sterilizing seeds, all the operation must be done on a clean bench.
Recipes
- 100 ml MS solid medium
0.44 g Murashige and Skoog basal medium powder
1 g sucrose
Dissolve in 90 ml ddH2O and adjust pH to 5.8-5.9 with KOH
Bring volume to 100 ml with ddH2O
Add 0.8 g agar powder and autoclave at 121 °C for 20 min - 10 ml 10 mM GA3 stock
34.638 mg GA3 powder
Dissolve in 10 ml absolute ethanol and filter with a 0.2 µm filter at a clean bench
Aliquot into sterile 1.5 ml Eppendorf tubes
Cover the tubes with aluminum foil to protect from light, store at -20 °C - 100 ml 70% ethanol
70 ml absolute ethanol
Bring volume to 100 ml with ddH2O - 7 ml 1% sodium hypochlorite
1 ml 7% sodium hypochlorite
Bring volume to 7 ml with ddH2O - Sterilized distilled water
Autoclave distilled water at 121 °C, 20 min
Acknowledgments
This protocol was adapted from the Xiaojing Wang Lab at the South China Normal University. This work was supported by the National Natural Science Foundation of China (grant No. 90917011 to X.W.). We thank Dr. craig schluttenhofer (University of Kentucky) for proofreading the article.
References
- Zhong, C., Xu, H., Ye, S., Wang, S., Li, L., Zhang, S. and Wang, X. (2015). Gibberellic acid-stimulated Arabidopsis6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol 169(3): 2288-2303.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhong, C., Xu, H., Ye, S., Zhang, S. and Wang, X. (2016). Arabidopsis Seed Germination Assay with Gibberellic Acid. Bio-protocol 6(22): e2005. DOI: 10.21769/BioProtoc.2005.
- Zhong, C., Xu, H., Ye, S., Wang, S., Li, L., Zhang, S. and Wang, X. (2015). Gibberellic acid-stimulated Arabidopsis6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol 169(3): 2288-2303.
Category
Plant Science > Plant biochemistry > Plant hormone
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link