- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Brainstem-spinal Cord Preparation from Newborn Rat
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2003 Views: 9774
Reviewed by: Soyun KimShai BerlinAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Sciatic Nerve Cut and Repair Using Fibrin Glue in Adult Mice
Erica T. Akhter [...] Francisco J. Alvarez
Sep 20, 2019 6003 Views

Optogenetic Approach for Investigating Descending Control of Nociception in Ex Vivo Spinal Cord Preparation
Volodymyr Krotov [...] Pavel Belan
Nov 5, 2025 1986 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1342 Views
Abstract
The brainstem-spinal cord preparation of newborn rat contains neural networks able to produce motor output in absence of sensory feedback. These neural structures, commonly called central pattern generators (CPGs), are involved in many vital functions such as respiration (Morin and Viala, 2002; Giraudin et al., 2008) or locomotion (Juvin et al., 2005). Here we describe a procedure for the isolation of the brainstem-spinal cord tissue of neonatal rat (0-2 days old). A surgical method under binocular microscope allows the brainstem and the spinal cord to be isolated in vitro and the motor outputs to be recorded. This preparation can then be used for diverse experimental approaches, such as electrophysiology, pharmacology or anatomical studies, and constitutes a useful model to study the interaction between CPGs (Juvin et al., 2007; 2012; Giraudin et al., 2012; Le Gal et al., 2014; 2016).
Background
Historically, the in vitro spinal cord of neonatal rodent was developed to study the spinal reflexes (Otsuka and Konishi, 1974). In 1984, Suzue was the first to develop the in vitro brainstem-spinal cord preparation of newborn rat. Thus, it was possible to demonstrate that an isolated central nervous system was able to generate spontaneously what is referred as fictive respiratory activity. Later, it was then possible to determine the location of the CPGs underlying the locomotor rhythm generation (Cazalets et al., 1995; Kjaerulff and Kiehn, 1996; Ballion et al., 2001) and those engaged in respiratory rhythm generation (Smith et al., 1991; Onimaru and Homma, 2003). In our research team, this preparation has been mainly used to study the neural mechanisms underlying the interaction between CPGs. For instance, in a context of interaction between CPGs involved in the same function, our results have contributed to characterize the role played by the sensory afferents and the spinal thoracic segments in the coordination between the cervical and the lumbar locomotor CPGs (Juvin et al., 2005; 2012). Similarly, this preparation allows studies on the neural mechanisms involved in coordination between CPGs engaged in different functions. Based on electrical stimulation of dorsal roots, it was shown that the proprioceptive inputs originating from both hindlimb and forelimb are involved in the respiratory rhythm entrainment observed during locomotion (Morin and Viala, 2002; Giraudin et al., 2012). These ascending entraining signals from the cervical and lumbar afferents are conveyed to the brainstem respiratory centers via a brainstem pontine relay located in the parabrachial/Kölliker-Fuse complex (Giraudin et al., 2012). Using pharmacological and intracellular (patch-clamp recording) approaches on the same preparation, recent results have demonstrated for the first time the existence of an ascending pathway from the lumbar locomotor CPGs to the respiratory CPGs. This central neurogenic mechanism, involving a substance P-dependent modulating mechanism, could play a crucial role in the increased respiratory frequency observed during locomotion (Le Gal et al., 2014). In addition, it was also demonstrated that the locomotor related signal from the lumbar locomotor CPGs selectively modulates the intracellular activity of spinal expiratory neurons (Le Gal et al., 2016). Altogether, our results obtained on the in vitro brainstem spinal cord preparation of new born rat have contributed to increase our understanding of the cellular bases engaged in the coordination of rhythmic neural circuitry responsible for different functions.
Materials and Reagents
- Needles (25 G) (Henke-Sass Wolf, catalog number: 4710005016 )
- Neonatal rat (0-2 days old)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911 )
- Sodium phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: S0751 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C5080 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S8875 )
- D-glucose (Thermo Fisher Scientific, Fisher Scientific, catalog number: AC410950010 )
- Sodium hydroxide (NaOH) (1 N) (Sigma-Aldrich, catalog number: 795429 )
- Hydrochloric acid (HCl) (37%) (Sigma-Aldrich, catalog number: 258148 )
- Isoflurane (Piramal Enterprises, Piramal HealthCare, catalog number: Isoflurane )
- Vaseline (Sigma-Aldrich, catalog number: 16415 )
- Artificial cerebro-spinal fluid (aCSF) solution (see Recipes)
Equipment
- FisherbrandTM beaker (1 L) (Thermo Fisher Scientific, Fisher Scientific, catalog number: 15449083 )
- Ice bucket (SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: M18848-2001 )
- Induction chamber for inhalational anesthesia (TemSega, catalog number: chamber )
- Isoflurane vaporizer (TemSega, model: Isoflurane vaporizer )
- Carbogen, 95% O2, 5% CO2 (The Linde Group, Linde Gaz, model: Carbogen B50 )
- Scalpel blade (LCH MEDICAL PRODUCTS, catalog number: SCX23 ) (Figure 1a)
- Small scissors (Moria, model: MC26B ) (Figure 1a)
- Fine forceps (Fine Science Tools, Dumont, model: 55 ) (Figure 1a)
- Dissection chamber (plastic box, 100 ml) with 2 mm layer of silicone elastomer
- Binocular microscope (Olympus, model: SZX7 )
- Recording chamber (10 ml) composed by a standard petri dish (60 mm) with 2 mm layer of silicone elastomer
- Peristaltic pump (Gilson, model: Minipuls® 3 )
Procedure
- Artificial cerebro-spinal fluid (aCSF)
For the preparation, see explanation in the Recipes section below. Once prepared, place a beaker containing aCSF in an ice bucket throughout experience. - Dissection and isolation of the brainstem-spinal cord
- Place a neonatal rat (0-2 days old) in an induction chamber for inhalational anesthesia. Animal is anesthetized with 4% isoflurane until the loss of reflex responsiveness to tail pinching (during 7 to 10 min).
- Using a scalpel, swiftly euthanize the animal by decapitation (incision must be performed in front of the ears) (Figure 1b). This allows decerebration just rostrally to the fifth cranial nerves.
- Remove the skin and muscles covering the back with forceps and scissors (Figure 1c).
- Transfer the preparation into a dissection chamber filled with aCSF at 4 °C and insert needle in each fore- and hind-limb to hold the preparation (slightly stretched) with the dorsal surface upwards (Figure 1d).
- Under binocular microscope, hold and lift up the skull bones with fine forceps. Using small scissors, cut the skull bones on both sides in order to expose the brainstem (Figure 1e).
- Using the same procedure along vertebral column, cut each vertebra on both sides to expose the spinal cord (dorsal laminectomy) (Figure 1f).
- Turn the preparation over in order to maintain its ventral surface upwards (Figure 1g).
- Hold and lift up the skull bones with fine forceps (Figure 1h) and cut all ventral and dorsal spinal roots leaving as much length as possible. The inset in Figure 1h shows how the skin, muscles and bones are removed to allow laminectomy to be performed. To avoid damage of brainstem and/or spinal cord, it is crucial to never hold nervous tissue with forceps.
- Using a spoon, carefully transfer the brainstem-spinal cord with its ventral and dorsal spinal roots still attached in a 10 ml recording chamber containing circulating aCSF (flow rate, 3-5 ml/min adjusted with the peristaltic pump) (Figure 1i).
- In order to ensure mechanical stability, the preparation is pinned down with its ventral surface upward by several pins inserted through the meninges surrounding the brainstem and the spinal cord.
- To assess the quality of the procedure, motor output activity in spinal ventral roots is recorded with glass suction electrodes filled with aCSF solution (Figure 2a). Signals (burst of action potentials) are amplified (10,000 times) by differential AC amplifiers (low cutoff, 100 Hz; high cutoff, 1 kHz), digitized and acquired via an analogical/digital interface, and finally stored on a computer. The dissection is successful when motor activities are spontaneously generated by the isolated preparation (Figures 2b-2c).
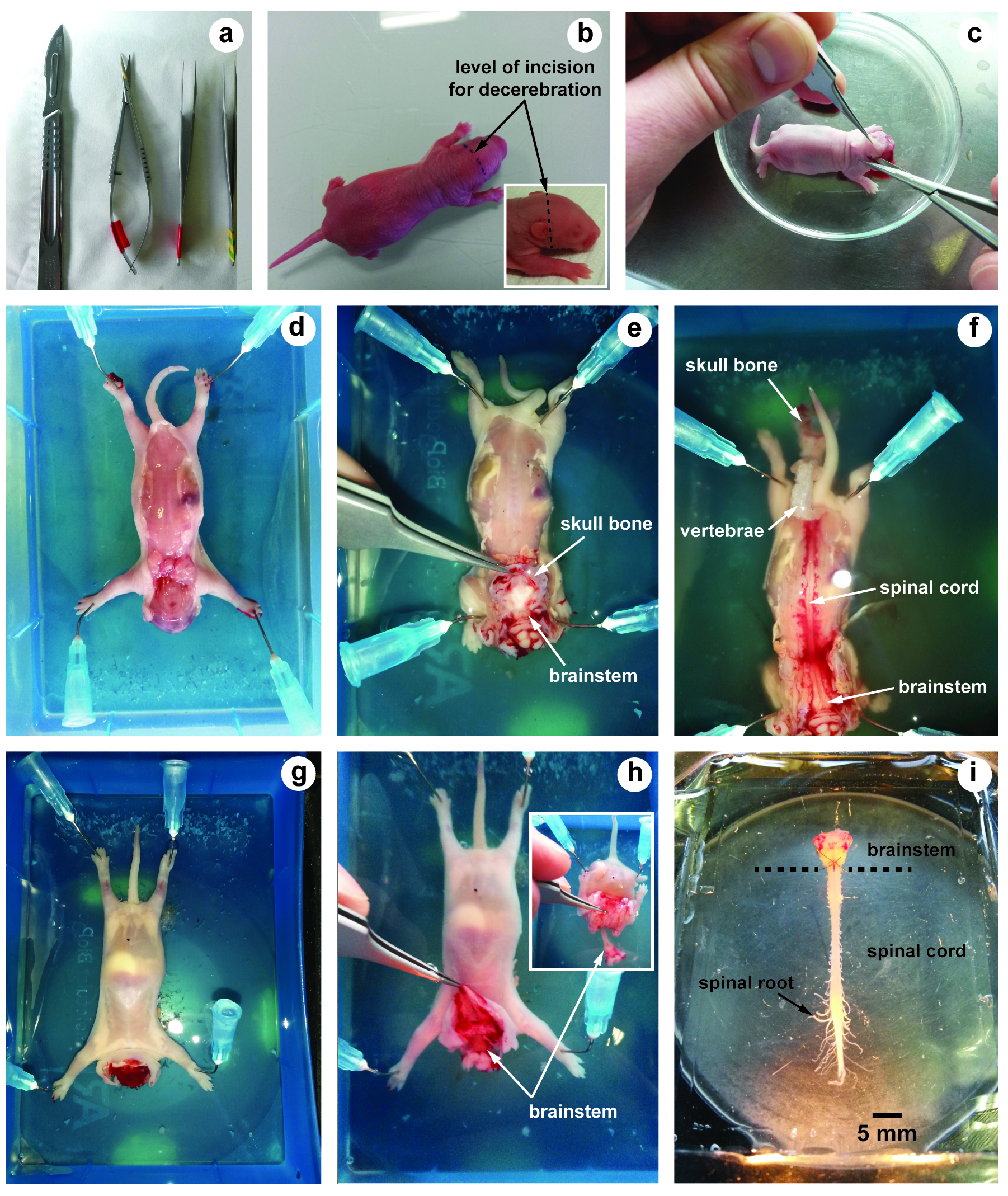
Figure 1. Illustrations of brainstem-spinal cord dissection. a. Tools used for dissection; b. Neonatal rat (2 days old) and level of incision for decerebration; c. After decerebration, skin and muscles covering the back are removed; d-f. The dorsal surface of the brainstem and the spinal cord is first exposed; g and h. The ventral surface; i. Image showing the isolated brainstem-spinal cord preparation with its spinal roots still attached.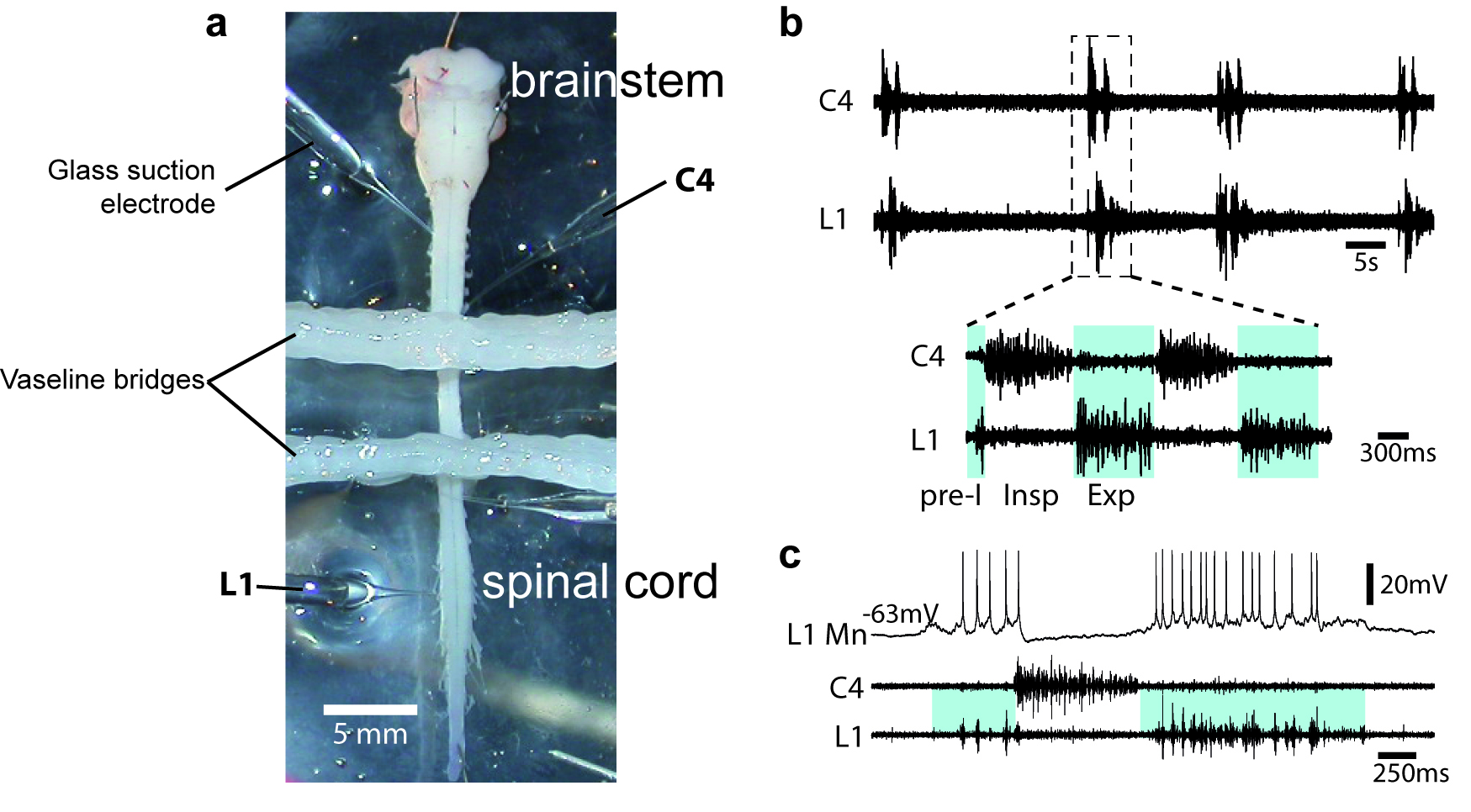
Figure 2. Motor output activity in spinal ventral roots from in vitro newborn rat preparation. a. Image of an isolated brainstem-spinal cord (from a P2 newborn animal) in a recording chamber. When required, the recording chamber is partitioned either 2 or 3 compartments with barriers of syringe-ejected Vaseline to permit differential exposure of selected spinal cord regions to pharmacological treatment. b. Simultaneous recordings are made from the 4th cervical (C4) and the 1st lumbar (L1) ventral roots. Respiratory-like rhythm is spontaneously generated, showing clear inspiratory (Insp) C4 and pre-inspiratory/expiratory (pre-I; Exp) L1 alternated phases. c. Blind techniques (with sharp or patch electrodes) were used to record rhythmic activity of brainstem and spinal respiratory neurons. Here, the firing of the impaled neuron is in synchrony with L1 ventral root discharge and therefore is identified as an expiratory motoneuron (MN).
- Place a neonatal rat (0-2 days old) in an induction chamber for inhalational anesthesia. Animal is anesthetized with 4% isoflurane until the loss of reflex responsiveness to tail pinching (during 7 to 10 min).
Data analysis
- Respiratory and locomotor-related activities in spinal ventral roots can be recorded using glass suction electrodes filled with aCSF solution. The motor outputs generated for these two functions consist in rhythmic bursting activities, allowing several types of analysis. Thus, by recording the respiratory motor outputs (see Giraudin et al., 2008), it is possible to determine the respiratory frequency before, during and after pharmacological or lesion experiments. In order to quantify the modulation of the respiratory rhythm between different conditions, statistical analyses are performed using dedicated software, such as SigmaPlot 11.0 (Systat). The results are expressed as mean ± SEM (or SD), and Student’s t-test is used to compare the means of two groups, whereas repeated measures ANOVA and subsequent Tukey’s post hoc tests are used to compare more than two groups. The differences are considered statistically significant when P ˂ 0.05.
- It is also possible to establish the phase relationship between motor-related bursting activities, such as respiratory vs. locomotor, or locomotor vs. locomotor bursting activities. In order to calculate the phase relationship, several burst activities of a given ventral root are selected and taken as reference. The phase values of burst onsets in a second ventral root are calculated by dividing the duration between the onsets of two consecutive burst onsets of each motor output by the reference cycle period. The phase values are then plotted on a circular phase diagram with a scale ranging from 0 to 1. A phase value approaching 0 or 1 indicates synchrony, whereas values close to 0.5 reflects alternation.
Recipes
- Artificial cerebro-spinal fluid (aCSF) solution
- Add 7.305 g NaCl (final concentration 100 mM), 0.249 g KCl (4 mM), 0.069 g NaH2PO4 (1.2 mM), 0.185 g CaCl2 (2 mM), 0.233 g MgCl2 (1.3 mM), 1.764 g NaHCO3 (25 mM), 5.406 g D-glucose (30 mM) in a beaker filled with 1 L of distilled water
- Mix the solution with a magnetic stirrer
- Place the beaker in an ice bucket and perfuse the aCSF with 95% O2 and 5% CO2 for at least 30 min before use
- Adjust the pH to 7.4 (use NaOH or HCl)
Acknowledgments
This protocol was adapted from our published paper: Le Gal et al. (2016), J-P. Le Gal and A. Nicolosi were supported by a doctoral studentship from the French “Ministère de l’Enseignement Supérieur et de la Recherche”. Authors thank R. Anselm for his comments and for English revision.
References
- Ballion, B., Morin, D. and Viala, D. (2001). Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci 14(10): 1727-1738.
- Cazalets, J. R., Borde, M. and Clarac, F. (1995). Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15(7 Pt 1): 4943-4951.
- Giraudin, A., Cabirol-Pol, M. J., Simmers, J. and Morin, D. (2008). Intercostal and abdominal respiratory motoneurons in the neonatal rat spinal cord: spatiotemporal organization and responses to limb afferent stimulation. J Neurophysiol 99(5): 2626-2640.
- Giraudin, A., Le Bon-Jego, M., Cabirol, M. J., Simmers, J. and Morin, D. (2012). Spinal and pontine relay pathways mediating respiratory rhythm entrainment by limb proprioceptive inputs in the neonatal rat. J Neurosci 32(34): 11841-11853.
- Juvin, L., Le Gal, J. P., Simmers, J. and Morin, D. (2012). Cervicolumbar coordination in mammalian quadrupedal locomotion: role of spinal thoracic circuitry and limb sensory inputs. J Neurosci 32(3): 953-965.
- Juvin, L., Simmers, J. and Morin, D. (2005). Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J Neurosci 25(25): 6025-6035.
- Juvin, L., Simmers, J. and Morin, D. (2007). Locomotor rhythmogenesis in the isolated rat spinal cord: a phase-coupled set of symmetrical flexion extension oscillators. J Physiol 583(Pt 1): 115-128.
- Kjaerulff, O. and Kiehn, O. (1996). Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16(18): 5777-5794.
- Le Gal, J. P., Juvin, L., Cardoit, L. and Morin, D. (2016). Bimodal respiratory-locomotor neurons in the neonatal rat spinal cord. J Neurosci 36(3): 926-937.
- Le Gal, J. P., Juvin, L., Cardoit, L., Thoby-Brisson, M. and Morin, D. (2014). Remote control of respiratory neural network by spinal locomotor generators. PLoS One 9(2): e89670.
- Morin, D. and Viala, D. (2002). Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J Neurosci 22(11): 4756-4765.
- Onimaru, H. and Homma, I. (2003). A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23(4): 1478-1486.
- Otsuka, M. and Konishi, S. (1974). Electrophysiology of mammalian spinal cord in vitro. Nature 252(5485): 733-734.
- Smith, C. J., Ellenberger, H. H., Ballanyi, K., Richter, D. W. and Feldman, J. L. (1991). Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726-729.
- Suzue, T. (1984). Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol 354: 173-183.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Le Gal, J., Nicolosi, A., Juvin, L. and Morin, D. (2016). In vitro Brainstem-spinal Cord Preparation from Newborn Rat. Bio-protocol 6(22): e2003. DOI: 10.21769/BioProtoc.2003.
-
Le Gal, J. P., Juvin, L., Cardoit, L. and Morin, D. (2016). Bimodal respiratory-locomotor neurons in the neonatal rat spinal cord. J Neurosci 36(3): 926-937.
Category
Neuroscience > Sensory and motor systems > Spinal cord
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












