- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Recombinant Mannitol-1-phosphate Dehydrogenase Activity from Ectocarpus sp.
Published: Vol 6, Iss 21, Nov 5, 2016 DOI: 10.21769/BioProtoc.1982 Views: 8904
Reviewed by: Valentine V TrotterYanjie LiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pyruvate:ferredoxin Oxidoreductase (PFR1) Activity Assays Using Methyl Viologen as Artificial Electron Acceptor
Jens Noth
Sep 5, 2013 12896 Views

Assay of the Carboxylase Activity of Rubisco from Chlamydomonas reinhardtii
Hemanth P. K. Sudhani [...] Joaquín Moreno
Dec 5, 2015 10408 Views

Determination of Recombinant Mannitol-1-phosphatase Activity from Ectocarpus sp.
Agnès Groisillier and Thierry Tonon
Aug 20, 2016 9069 Views
Abstract
Brown algae belong to a phylogenetic lineage distantly related to green plants and animals, and are found predominantly, but not exclusively, in the intertidal zone, a harsh and frequently changing environment. Because of their unique evolutionary history and of their habitat, brown algae feature several peculiarities in their metabolism. One of these is the mannitol cycle, which plays a central role in their physiology, as mannitol acts as carbon storage, osmoprotectant, and antioxidant. This polyol is derived directly from the photoassimilate fructose-6-phosphate via the action of a mannitol-1-phosphate dehydrogenase (M1PDH, EC 1.1.1.17) and a mannitol-1-phosphatase (M1Pase, EC 3.1.3.22). This protocol describes the biochemical characterization of the recombinant catalytic domain of one of the three M1PDHs identified in Ectocarpus sp. This recombinant catalytic domain, named hereafter M1PDHcat, catalyzes the reversible conversion of fructose-6-phosphate (F6P) to mannitol-1-phosphate (M1P) using NAD(H) as a cofactor. M1PDHcat activity was assayed in both directions i.e., F6P reduction and M1P oxidation (Figure 1).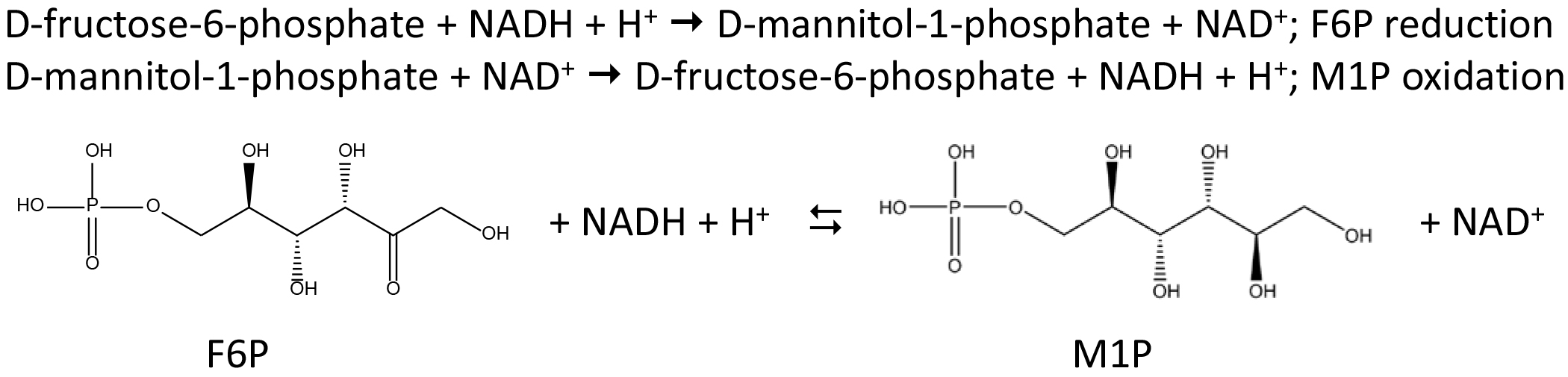
Figure 1. Reversible reaction of mannitol-1-phosphate dehydrogenase
Materials and Reagents
- UV-Star® PS microplate (96 well) (Greiner Bio One International, catalog number: 655801 )
- 0.22 µm filter
- Purified recombinant His-tagged M1PDHcat
Note: This protein was produced in Escherichia coli BL21 (DE3) containing the recombinant pFO4_M1PDHcat vector, as described by Groisillier et al. (2010). This recombinant protein was purified by affinity chromatography using a HisPrep FF 16/10 column (GE Healthcare) and then by gel filtration using a Superdex 200 (GE Healthcare) onto an Äkta avant system (GE Healthcare). The complete purification protocol is described in details in Bonin et al. (2015). - MilliQ water
- Trizma® base (Sigma-Aldrich, catalog number: T1503 )
- 4-morpholineethane-sulfonic acid (MES) (Sigma-Aldrich, catalog number: M2933 )
- HEPES (Sigma-Aldrich, catalog number: H3375 )
- Bis-Tris propane (Sigma-Aldrich, catalog number: B6755 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 71380 )
- Examples of chemicals to be tested to assess substrate and co-factor specificity:
- D-mannitol-1-phosphate (Sigma-Aldrich, catalog number: 92416 )
- D-fructose-1-phosphate (Sigma-Aldrich, catalog number: F1127 )
- α-D-glucose-1-phosphate disodium salt hydrate (Sigma-Aldrich, catalog number: G9380 )
- D-mannose-6-phosphate sodium salt (Sigma-Aldrich, catalog number: M3655 )
- D-glucose-6-phosphate sodium salt (Sigma-Aldrich, catalog number: G7879 )
- D-fructose-6-phosphate disodium salt hydrate (Sigma-Aldrich, catalog number: F3627 )
- β-NAD (Sigma-Aldrich, catalog number: N1636 )
- β-NADP (Sigma-Aldrich, catalog number: N5755 )
- β-NADH (Sigma-Aldrich, catalog number: N8129 )
- β-NADPH (Sigma-Aldrich, catalog number: N5130 )
- 1 M Tris-HCl (see Recipes)
- 5 M NaCl (see Recipes)
- 10 mM NADH (see Recipes)
- 10 mM NAD+ (see Recipes)
Equipment
- NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, model: NanoDrop 2000 )
- Safire2 UV spectrophotometer microplate reader (Tecan Trading)
Software
- Hyper 32 (Informer Technologies, model: hyper32)
- Microsoft Excel
Procedure
- The standard F6P reduction reaction mixture contains 50 mM Tris-HCl (pH 7.0), 1 mM F6P, 0.2 mM NADH and 0.1 to 10 µg of purified recombinant M1PDHcat, in a final volume of 100 µl. The standard M1P oxidation reaction mixture contains 50 mM Tris-HCl (pH 9.0), 1 mM M1P, 0.5 mM NAD+ and 0.1 to 10 µg of purified recombinant M1PDHcat in 100 µl. Quantities of proteins are determined with NanoDrop at 280 nm based on the extinction coefficient of 37,525 M-1 cm-1 calculated for the protein of interest. The negative control corresponds to the reaction mixture in which substrate is substituted by MilliQ water (Table1).
Table 1. Composition of negative control and reaction mixture for determination of M1PDHcat activity in the direction of F6P reduction and M1P oxidation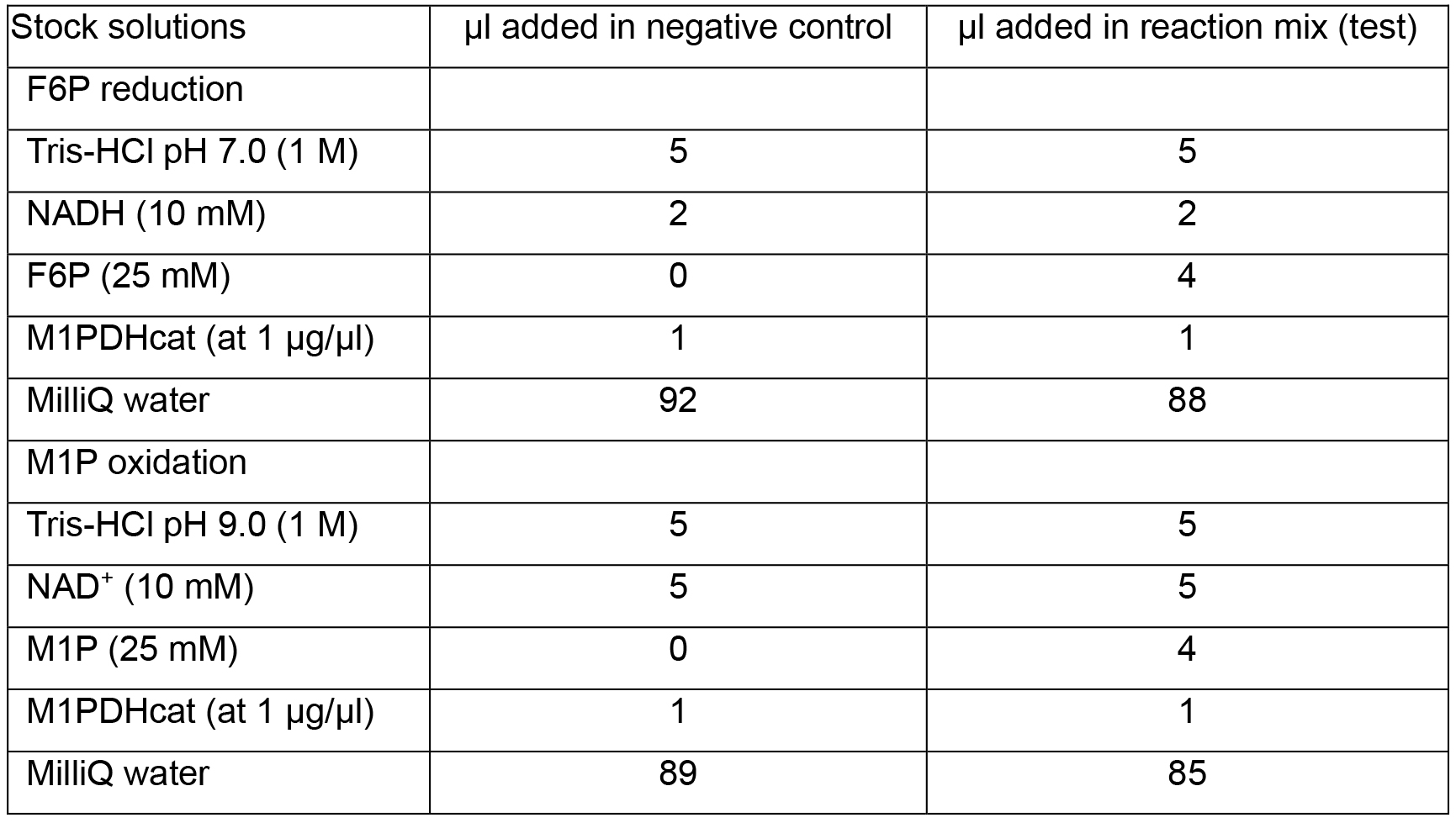
- The mix is prepared with water, Tris-HCl, NAD(H) and enzyme, then the reaction is started by adding the substrate and the activity is monitored by following changes in absorbance at 340 nm, which corresponds to the consumption (F6P • M1P) or production (M1P • F6P) of NADH, in a Safire2 UV spectrophotometer microplate reader. The plate is subjected to 10 sec of orbital shaking before measurements. Values are recorded every 13 sec approximately, and this interval of time can be modified according to the activity of the enzyme under consideration. The assay is performed at 30 °C for up to 20 min. Only the early linear part of the curves is used to calculate activity (Figures 2 and 3).
- F6P reduction and M1P oxidation activities, based on consumption or production of NADH respectively, are calculated using the formula:
[(ΔA340 nm test - ΔA340 nm negative control) x 0.0001/(t x 6220 x 0.3)]
Where,
ΔA340 nm = variation of absorbance during the duration of incubation,
T = duration of incubation (min),
6220 = extinction coefficient for NADH (L mol-1 cm-1),
0.3 = optical path (cm). This value depends of the plate/cuvette used,
0.0001 = assay volume (L),
One unit (U) of activity, F6P reduction or M1P oxidation, corresponds to 1 µmole of co-factor oxidized or reduced per min respectively.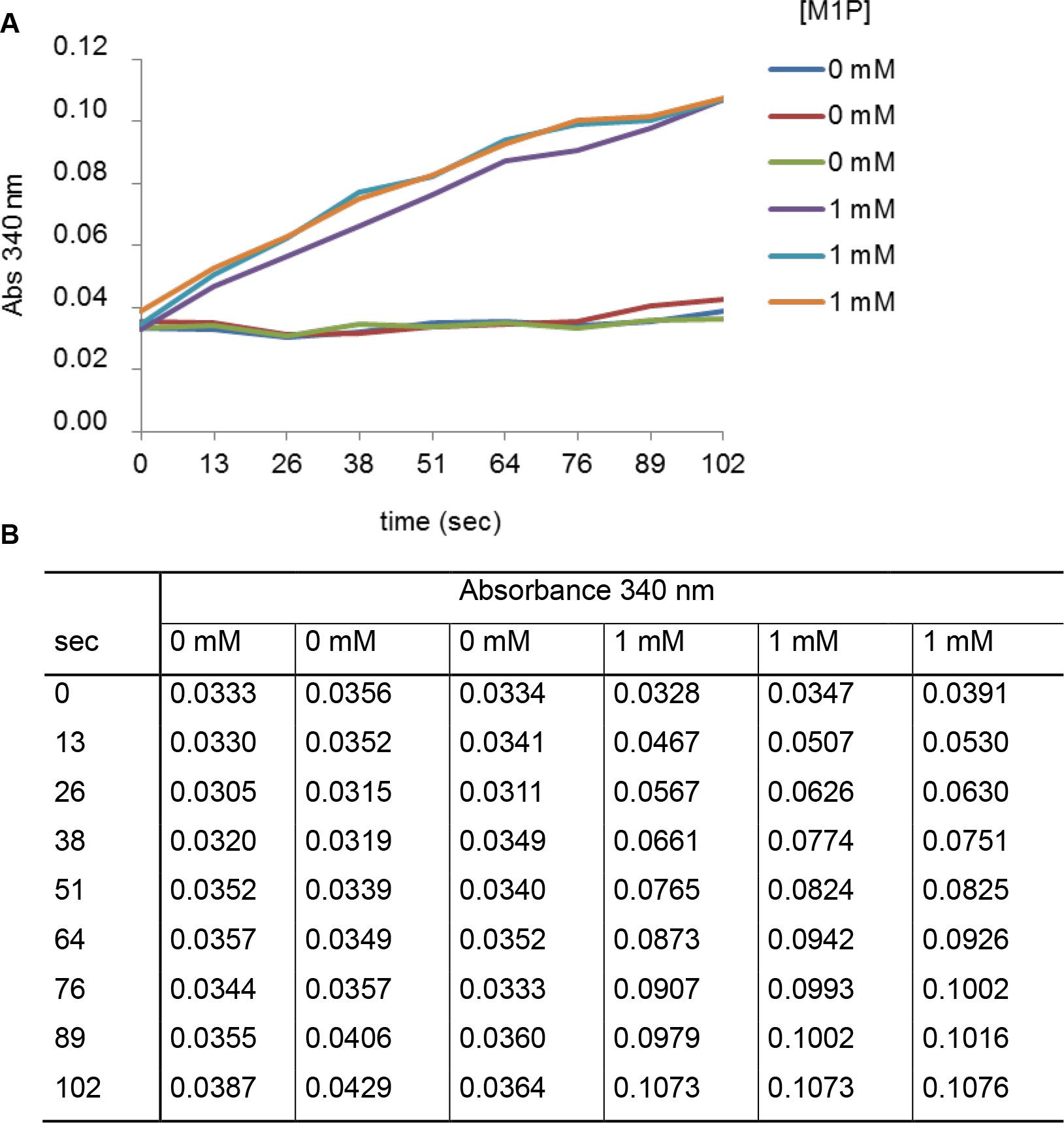
Figure 2. Measurement of absorbance (340 nm) monitored as a function of time (sec) in presence or in absence of M1P. A. The curves represent two series of triplicates obtained in absence or in presence of 1 mM of M1P and 0.2 µg of enzyme. B. The table contains values of absorbance measured under both conditions tested and at different times of experiment.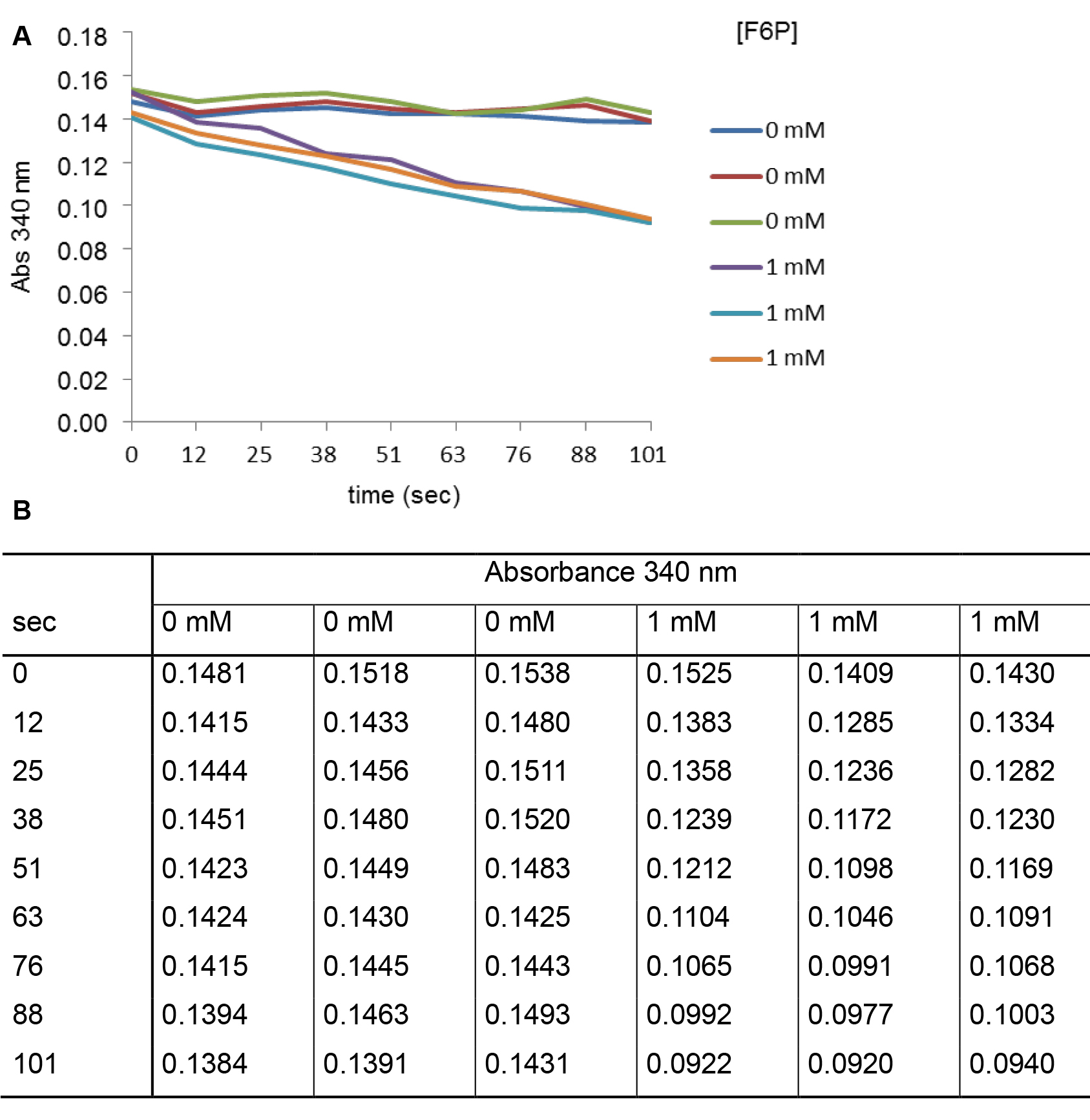
Figure 3. Measurement of absorbance (340 nm) monitored as a function of time (sec) in presence or in absence of F6P. A. The curves represent two series of triplicates obtained in absence or in presence of 1 mM of F6P and 0.04 µg of enzyme. B. The table contains values of absorbance measured under both conditions tested and at different times of experiment. - To calculate specific activities, divide the values obtained in the equation above by the quantity of M1PDHcat proteins present in the sample. Perform three replicates for each condition, and use the average calculated in absence and in presence of substrate to determine the specific activity of each reaction. This applies also to the experiments described below. From values of Figures 2 and 3, specific activities of M1PDHcat are 11.3 U/mg in presence of 1 mM M1P and 39.0 U/mg in presence of 1 mM F6P.
- To determine substrate specificity, test M1PDHcat activity in the presence of each substrate listed in the ‘Materials and Reagents’ section, using concentration ranging from 1 mM to at least 50 mM, at room temperature.
- To determine the optimal temperature, incubate the reaction mixtures used in step 1 at temperatures ranging, for instance, from 10 °C to 50 °C, with incremental of 5 °C or 10 °C. The experiments described below are performed at the optimal temperature of 30 °C.
- To determine the optimal pH, replace the 50 mM Tris-HCl pH 7.0 or pH 9.0 buffer used in step 1 by other buffers prepared at different pHs. As an indication, it is possible to use:
- 50 mM MES for pH 5.5, 6, 6.5
- 50 mM Bis-Tris propane for pH 6.5, 7, 7.5, 8, 8.5, 9, 9.5
- 50 mM Tris-HCl for pH 7, 7.5, 8, 8.5, 9
- 50 mM Tris-acetate for 6, 6.5, 7
- 50 mM HEPES for 7, 7.5, 8, 8.5
- To examine the influence of NaCl, add NaCl to the reaction mixture described in step 1 to obtain final concentrations ranging from 0 to 1 M.
- To estimate the kinetic parameters of the enzyme for a selected substrate S, run individual enzyme reactions in the presence of at least five different concentrations of this substrate (for example 0.1 mM, 0.25 mM, 1 mM, 4 mM, 20 mM final concentration), and a fixed concentration of NAD+ or NADH. Determine the initial reaction rate for each reaction and plot 1/V versus 1/[S] to obtain a Lineweaver-Burk plot, from which Km and Vm for S can be calculated (Figure 4) using the hyper 32 software.
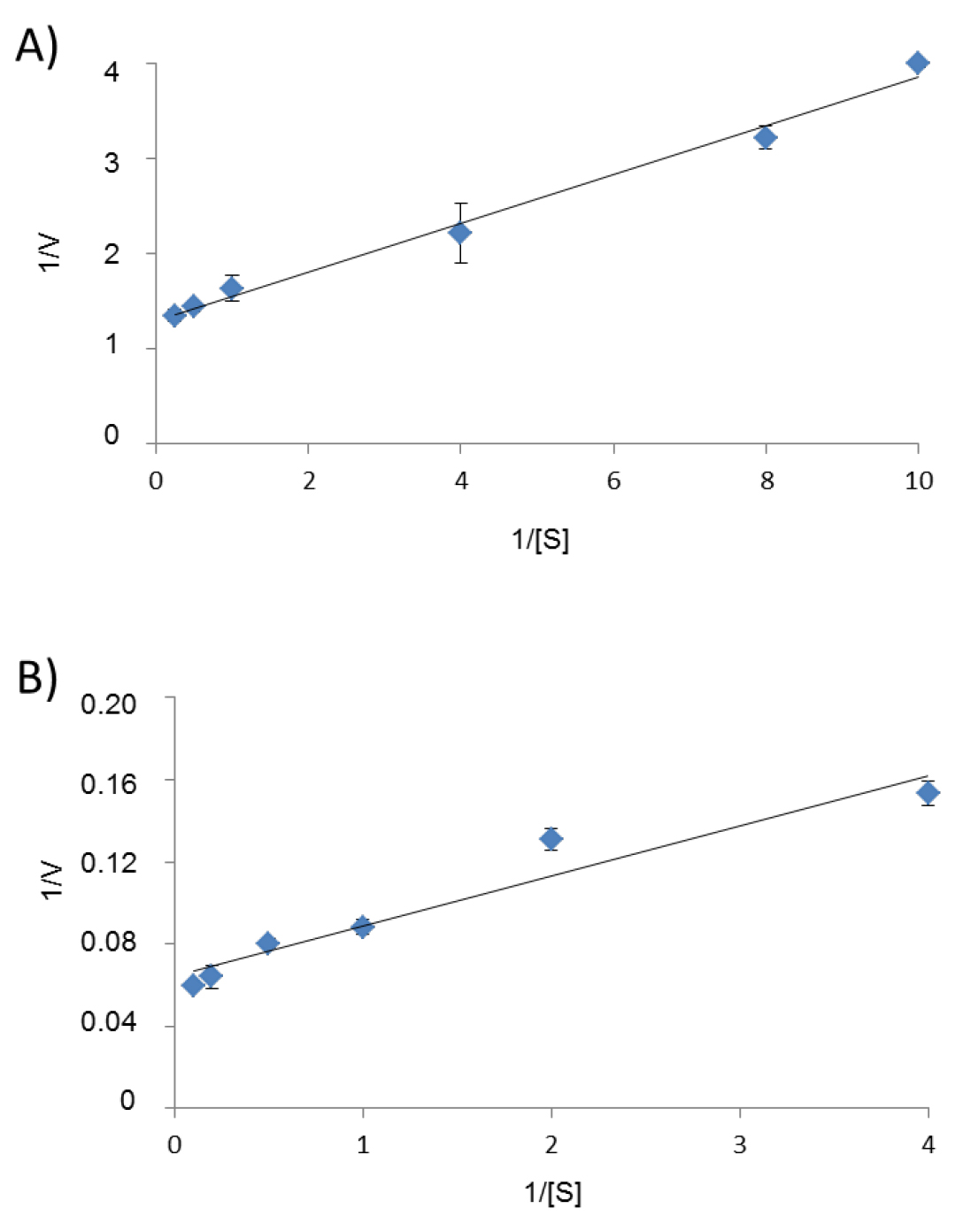
Figure 4. Lineweaver-Burk plots used to determine in A) the Km (0.19 mM) and Vm (46 U/mg) of M1PDHcat for F6P and in B) the Km (0.38 mM) and Vm (15.6 U/mg) of M1PDHcat for the M1P. [S] is the substrate concentration (in mM) and V is the reaction rate (in U/mg of protein). Three replicates were performed for each assay.
Notes
- Before determining Km and Vm for a given substrate S, be sure that cofactor is in excess, i.e., that V does not increase with increasing quantities of cofactor in the reaction mixture; in the same vein, before determining Km and Vm for the cofactor, be sure that S is in excess, i.e., that V does not increase with increasing quantities of S in the reaction mixture. The theory is that this saturating concentration is equivalent to 100Km, but 10Km is usually enough (Bisswanger, 2014). It is then necessary to adjust the amount of M1PDHcat and the incubation time to obtain a linear variation of absorbance at 340 nm, i.e., a linear change of NADH quantity.
Recipes
- 1 M Tris-HCl
Dissolve 121.14 g of Trizma® base in about 800 ml of MilliQ water, adjust pH with HCl then complete to 1 liter. Filter through a 0.22 µm filter and store at room temperature. - 5 M NaCl
Dissolve 95.21 mg of NaCl in 10 ml of MilliQ water. Filter through a 0.22 µm filter and store at room temperature. - 10 mM NADH
Dissolve 6.63 mg of NADH in 1 ml of MilliQ water. Filter through a 0.22 µm filter and store at room temperature. Prepare fresh on the day of use. - 10 mM NAD+
Dissolve 6.63 mg of NAD+ in 1 ml of MilliQ water. Filter through a 0.22 µm filter and store at room temperature. Prepare fresh on the week of use and store at -20 °C.
Acknowledgments
This protocol was performed by Bonin et al. (2015). This work was supported by the French National Research Agency via the investment expenditure program IDEALG (ANR-1 0-BTBR-02). The authors also acknowledge funding from the Émergence-UPMC-2011 research program.
References
- Bisswanger, H. (2014). Enzymes assays. Perspec Sci 1: 41-55.
- Bonin, P., Groisillier, A., Raimbault, A., Guibert, A., Boyen, C. and Tonon, T. (2015). Molecular and biochemical characterization of mannitol-1-phosphate dehydrogenase from the model brown alga Ectocarpus sp. Phytochemistry 117: 509-520.
- Groisillier, A., Herve, C., Jeudy, A., Rebuffet, E., Pluchon, P. F., Chevolot, Y., Flament, D., Geslin, C., Morgado, I. M., Power, D., Branno, M., Moreau, H., Michel, G., Boyen, C. and Czjzek, M. (2010). MARINE-EXPRESS: taking advantage of high throughput cloning and expression strategies for the post-genomic analysis of marine organisms. Microb Cell Fact 9: 45.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Groisillier, A. and Tonon, T. (2016). Determination of Recombinant Mannitol-1-phosphate Dehydrogenase Activity from Ectocarpus sp.. Bio-protocol 6(21): e1982. DOI: 10.21769/BioProtoc.1982.
Category
Plant Science > Phycology > Protein > Activity
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











