- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Molecular Structures of Condensed Tannins from Plant Tissues Using HPLC-UV Combined with Thiolysis and MALDI-TOF Mass Spectrometry
Published: Vol 6, Iss 20, Oct 20, 2016 DOI: 10.21769/BioProtoc.1975 Views: 12293
Reviewed by: Arsalan DaudiElizabeth LibbyRebecca Van Acker

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1956 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1181 Views
Abstract
Condensed tannins extracted from plant tissues are suitable substitutes for phenolic resins. Their molecular structure, which might influence their chemical reactivity, can be assessed by the use of both HPLC-UV after acid thiolysis and MALDI-TOF mass spectrometry. Thiolysis of plant extracts in acidic methanol with cysteamine hydrochloride results in the release of the monomeric units of the condensed tannin oligomers that can be further quantified by reversed-phase HPLC-UV by comparison with analytical standards. MALDI-TOF mass spectrometry using 2,5-dihydroxybenzoic acid as matrix and K+ as cationization agent highlights the molecular structural characteristics (e.g., monomeric unit sequence) of the tannin oligomers. The methodologies permit the estimation of the mean and the maximum (observable) degree of polymerization, the type of monomeric units and the presence of glycosylation and/or esterification of the tannin oligomers.
Keywords: Condensed tanninsBackground
Condensed tannins are polyphenolic oligomers made of flavan-3-ol monomeric units that could be extracted from several plant tissues (e.g., softwood barks). They have been recognized as suitable alternatives to synthetic phenolics in resin formulations such as wood adhesives and foamed materials. The most common flavan-3-ol monomers detected in condensed tannins, which differ in the hydroxylation pattern and stereochemistry, are shown in Figure 1.
Figure 1. Most common monomers identified in the structure of condensed tannins
The specific structure of monomeric units in the oligomer and the polymerization degree strongly influence the chemical reactivity and physical properties of the tannins, e.g., condensation reaction rate with aldehydes, heavy metal chelation ability, and viscosity of aqueous solutions (Pizzi and Stephanou, 1994; Yoneda and Nakatsubo, 1998; Garnier et al., 2001). Identification of the molecular structure of the tannins is therefore important to better determine their possible exploitation.
The analysis of the structure of condensed tannins monomers has been performed with different methodologies, e.g., size exclusion chromatography (SEC), normal and reversed-phase high-performance liquid chromatography (HPLC), matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) and nuclear magnetic resonance (NMR) (Hümmer and Schreier, 2008).
Depolymerisation of tannins in acidic methanol and in the presence of toluene-α-thiol or cysteamine hydrochloride (thiolysis) followed by reversed-phase HPLC-UV was recognised as a suitable method for the estimation of the mean degree of polymerization of condensed tannins and the configuration of their building units (Matthews et al., 1997; Cheynier et al., 2001; Jerez et al., 2007; Bianchi et al., 2015). As well, MALDI-TOF MS has been shown to be successful in the determination of the structure of condensed tannins (Pasch et al., 2001; Monagas et al., 2010; Bianchi et al., 2014).
The proposed method for the analysis of condensed tannins extracted from plant tissues represents an optimization of the previously published procedures for HPLC-UV after acid thiolysis and MALDI-TOF MS. A careful tuning of the mobile phase gradient in the HPLC analysis was performed in order to reach a sufficient separation between the released flavanols and their corresponding thioethers. The choice of the cationization agent in the preparation of the sample for MALDI-TOF MS was also carefully gauged, especially in consideration of samples containing a relatively high amount of inorganic compounds like bark extracts.
The method was successfully used in the characterization of condensed tannins extracted from the bark of softwood species, e.g., Silver fir, European larch, Norway spruce, Douglas fir and Scots pine (Bianchi et al., 2015).
Materials and Reagents
- 1 ml Eppendorf tubes
- 2 ml Eppendorf tubes
- HPLC-filters 17 mm, PTFE, 0.45 µm (infochroma, catalog number: 8817-P-4 )
- Cosmosil Protein-R ø4.6 x 250 mm HPLC column (NACALAI TESQUE, catalog number: 06527-11 )
- Dry extracts from plant tissues (e.g., softwood bark)
The dry plant extracts could be obtained through different processes. For the extraction of tannins, the maceration of fine milled tissue (< 1 mm in size) in solvents like methanol, acetone or water is suggested. The extraction temperature should be between 30 and 90 °C, and the extraction time between 10 and 60 min. The drying of the extract has to be performed avoiding as much as possible post-modification (e.g., oxidation) of the extracts. Vacuum drying and/or freeze-drying are therefore recommended. After drying the extracts should be stored in a refrigerator (3-8 °C), protected from light and air. - Deionized water (18.2 MΩ-cm)
- 2,5-dihydroxybenzoic acid (matrix substance for MALDI MS, HPLC grade) (Sigma-Aldrich, catalog number: 85707 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Cysteamine hydrochloride (HPLC grade) (Sigma-Aldrich, catalog number: 49705 )
- Hydrochloric acid (HCl) (37%) (Sigma-Aldrich, catalog number: 320331 )
- Methanol (for HPLC) (Sigma-Aldrich, catalog number: 34860 )
- Acetone (for HPLC) (Sigma-Aldrich, catalog number: 270725 )
- Trifluoracetic acid (TFA) (Sigma-Aldrich, catalog number: 302031 )
- Acetonitrile (ACN) (Sigma-Aldrich, catalog number: 34998 )
- Flavan-3-ol analytical standards:
(+)-Catechin (Sigma-Aldrich, catalog number: 43412 )
(-)-Epicatechin (Sigma-Aldrich, catalog number: 68097 )
(-)-Gallocatechin (Sigma-Aldrich, catalog number: 01338 )
(-)-Epigallocatechin (Sigma-Aldrich, catalog number: 08108 ) - Mass spectra calibration standard covering a range from about 750 to 4000 m/z (e.g., Peptide calibration standard II, Bruker Daltonics, Germany)
- Thiolysis media (see Recipes)
- HPLC mobile phases (see Recipes)
Equipment
- Eppendorf pipette 1-10 µl
- Eppendorf pipette 100-1,000 µl
- HPLC system equipped with a UV-photodiode array detector. In our study an Agilent 1100 LC system was used (Agilent, Waldbronn).
- Water bath (Polyscience, UK)
- Ultrasonic bath (BANDELIN electronic, model: Sonorex RK510 )
- MALDI-TOF mass spectrometer equipped with laser emitting in UV wavelength. In our study a MALDI-TOF mass spectrometer Reflex III was used (Bruker Daltonics, Germany).
- Analytical balance
- Refrigerator
- HPLC vials (1 ml)
Procedure
- HPLC-UV after thiolysis
- Sample and standard preparation
- Dissolve the dried plant extracts (5 mg) in methanol (5 ml) and sonicate (35 kHz, 250W) at room temperature for 30 min.
- Transfer with an Eppendorf pipette 200 μl of the solubilized extracts in an Eppendorf tube (2 ml) and mix it with 200 μl of thiolysis media.
- Put the Eppendorf tube in the water bath set at 65 °C for 1 h to carry on the thiolysis. No shaking of the sample is needed. During the thiolysis the flavan-3-ol monomeric units of the condensed tannins are released in their native state (terminal units) as well as their respective thioethers (extension units), as schematically depicted in Figure 2.
Note: The thiolysis is effective only if condensed tannins are made of catechin and/or gallocatechin units. If tannins are made of fisetinidol, robinetinidol or monomeric units different than flavan-3-ols, the interflavonyl bond will not be cleaved.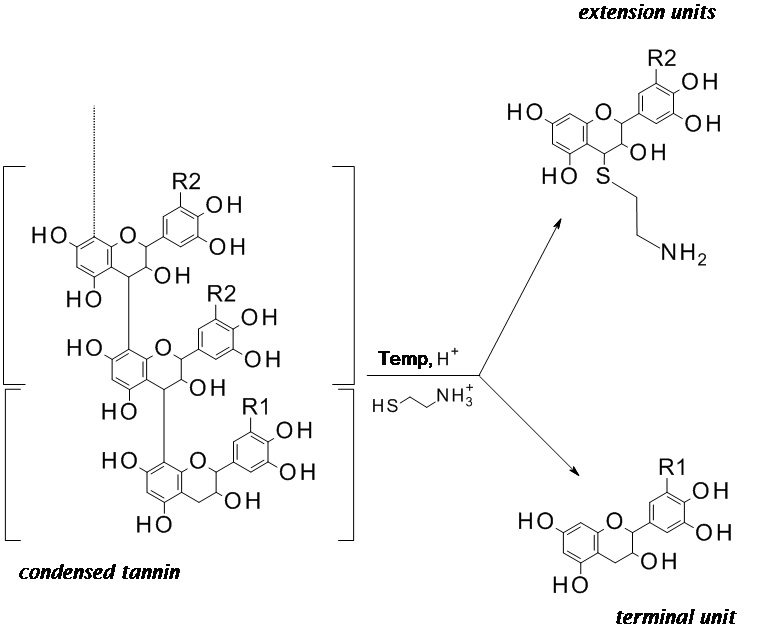
Figure 2. Scheme of the condensed tannin thiolysis with cysteamine hydrochloride. R1, R2 = H or OH. - The thiolysis is quenched with 1 ml of water and the solution is purified through a PTFE 0.45 μm HPLC filter.
- Non-thiolyzed reference samples are prepared by dissolving 200 μl of the solubilized extracts in sequence with 200 μl of methanol and 1.0 ml of water, followed by the same thermal treatment as used during the thiolysis (1 h at 65 °C) and purified through a PTFE 0.45 µm HPLC filter.
- Prepare calibration stock solutions of the flavan-3-ols (0.01, 0.02, 0.05, 0.10, 0.25, 0.50 g/L in methanol). The calibration standards solutions for HPLC are then prepared by diluting 200 μl of the calibration stock solutions in sequence with 200 μl of methanol and 1.0 ml of water. The calibration standard solutions are then thermally treated (1 h at 65 °C in a water bath) and purified (PTFE 0.45 µm HPLC-filter) in the same way as the samples.
- All samples and calibration standards are sealed in an HPLC vial and stored in a refrigerator between 3 and 8 °C until analysis. The samples remain stable for about 1 week under these conditions.
- Dissolve the dried plant extracts (5 mg) in methanol (5 ml) and sonicate (35 kHz, 250W) at room temperature for 30 min.
- Measurement
- Condition the reversed-phase HPLC-UV system equipped with the Cosmosil column using solution A (see Recipes) at 30 °C.
- Inject 25 μl of the sample (e.g., thiolyzed extract, non-thiolyzed extract, calibration standard) in the HPLC column. Before injection the samples should be conditioned at room temperature for at least 1 h. However, it is recommended to limit the exposition of the sample at room temperature to less than 24 h before the HPLC measurement.
- The mobile phase gradient described in Table 1 was applied at a flow equal to 1 ml/min. Compositions of solution A and solution B are described in the section Recipes. The temperature of the column is kept at 30 °C all over the measurement.
Table 1. Mobile phase gradient applied during HPLC measurements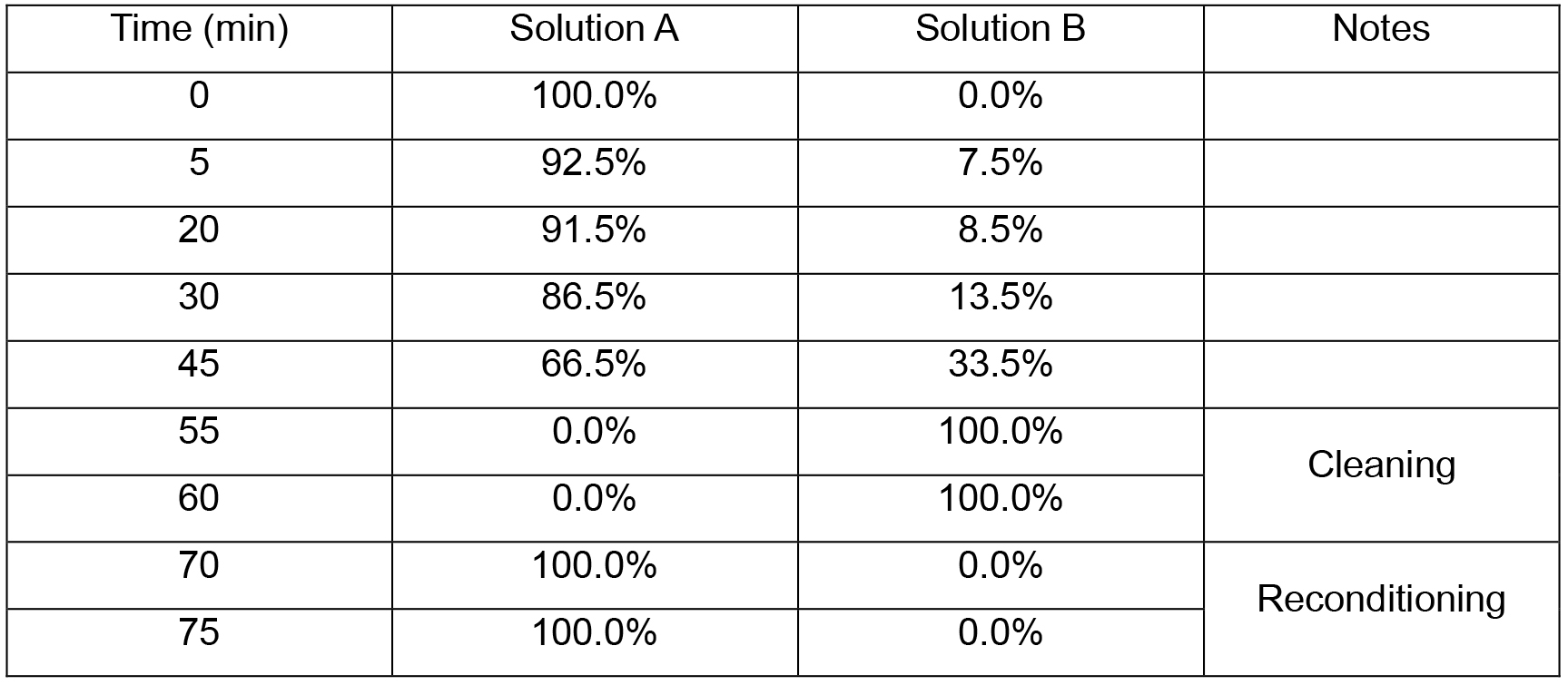
Notes: - The quasi-isocratic conditions between 5 and 20 min are needed to achieve a sufficient resolution (separation) of the peaks corresponding to epigallocatechin, catechin and their thioethers.
- The HPLC measurements should be repeated at least twice on replicated samples, therefore taking into account independent sample preparation, thiolysis, and analysis.
- Detect the absorbance at 280 nm. An example of the HPLC chromatograms at 280 nm of bark extracts before and after thiolysis is reported in Figure 3.
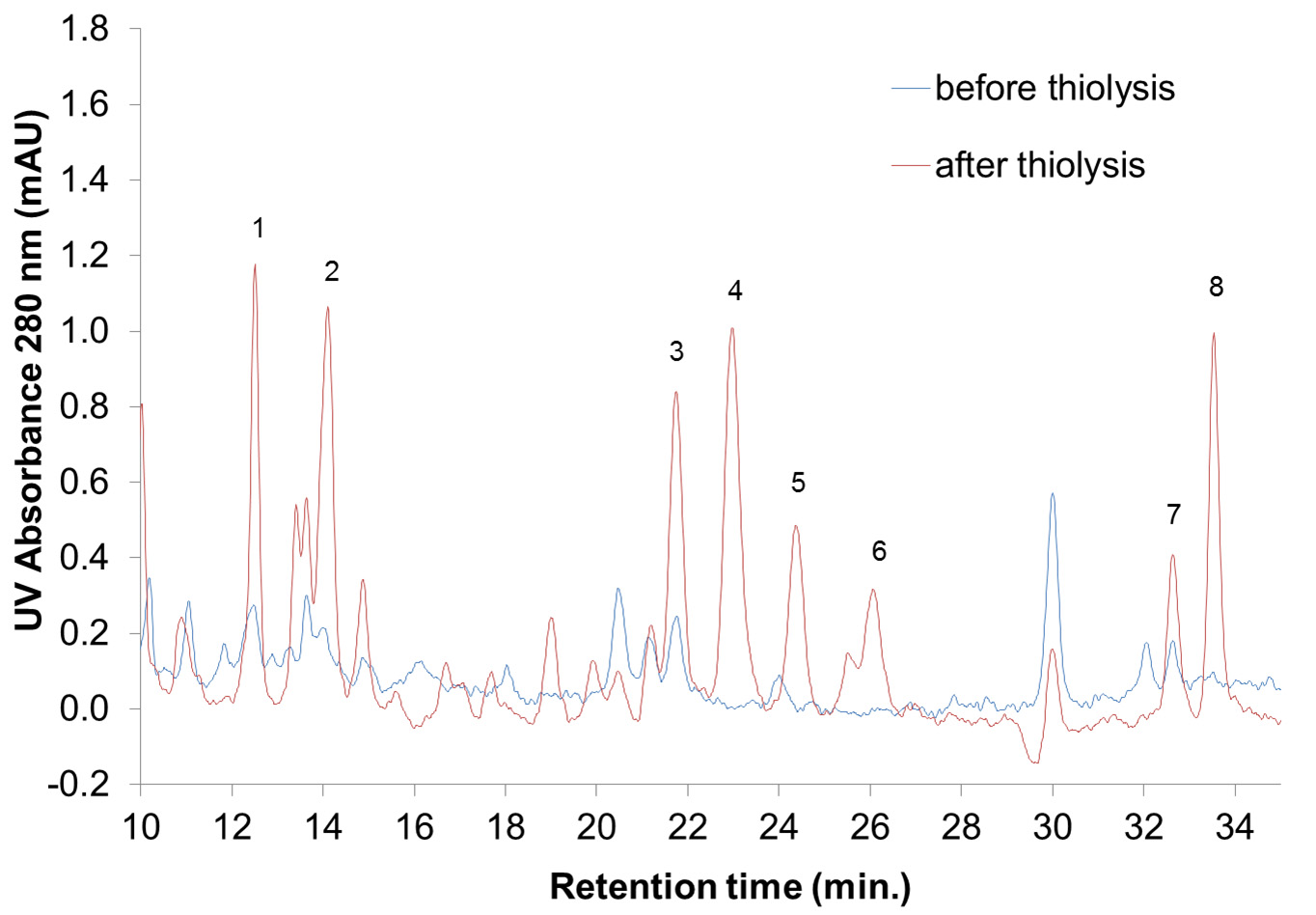
Figure 3. HPLC-UV chromatograms between 10 and 35 min of Silver fir (Abies alba [Mill.]) bark extracts before and after thiolysis. Absorption peaks are associated to various flavanol and flavanol thioethers as follows: 1 = gallocatechin; 2 = gallocatechin thioether; 3 = epigallocatechin; 4 = catechin; 5 = catechin thioether; 6 = epigallocatechin thioether; 7 = epicatechin; 8 = epicatechin thioether. - Integrate the area under the absorption peak(s) that corresponds to the flavan-3-ols and their thioethers.
Notes:- While analytical standards of native flavan-3-ols are commercially available from chemical substance distributors (e.g., Sigma-Aldrich), no standards are available for flavan-3-ol thioethers. The preparation of flavan-3-ol thioethers standards could be performed only starting from oligomers of known composition (also not easily available) and requires lengthy purification steps (Torres and Bobet, 2001). In the present study the retention times of the flavan-3-ol thioethers were identified by extemporary HPLC-MS measurements performed in an external laboratory using the same column and method and Agilent 1290 Infinity HPLC system equipped with a mass detector (Agilent 6130 quadrupole MS).
- Quantify the concentration of the flavan-3-ols in the thiolyzed and non-thiolyzed samples by comparison with the calibration curves developed for each single flavan-3-ol measuring the corresponding calibration standards. As already mentioned, no calibration standard are commercially available for the flavan-3-ol thioethers. Extemporary measurements performed on epicatechin dimers (proanthocyanidin B2) showed that, after complete thiolysis (complete disappearing of the peak corresponding to the epicatechin dimer) the peaks corresponding to epicatechin and epicatechin-thioether had almost an equal absorption area. The concentration of all flavan-3-ol thioethers was then calculated using for the flavanol thioethers the same UV molar absorption (MABS) of the corresponding native flavan-3-ols (e.g., MABS of epicatechin thioether was considered equal to MABS of epicatechin). In Table 2 the MABS for the measured flavan-3-ols are reported.
Table 2. Molar absorption factor (MABS) of flavan-3-ols and flavan-3-ol thioethers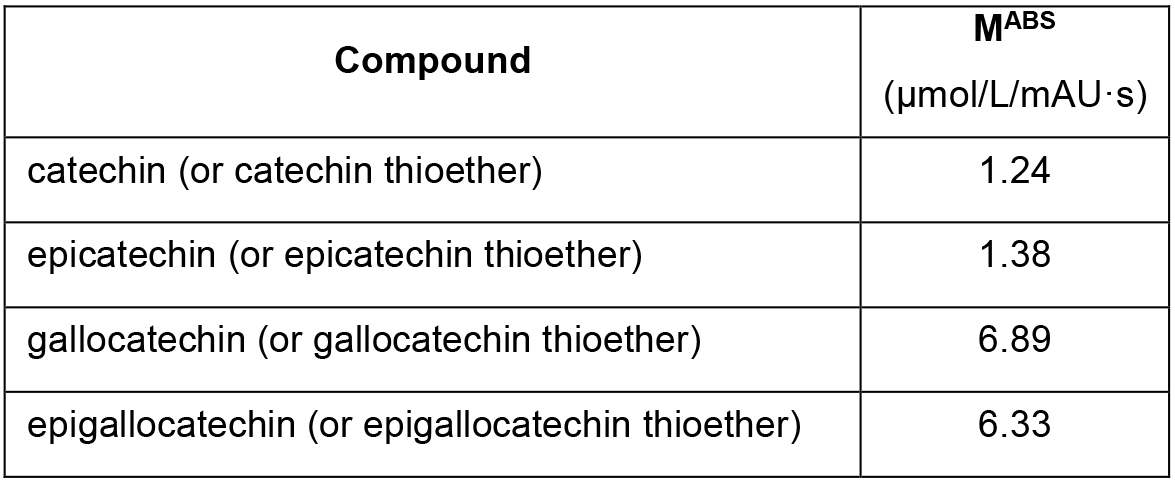
- While analytical standards of native flavan-3-ols are commercially available from chemical substance distributors (e.g., Sigma-Aldrich), no standards are available for flavan-3-ol thioethers. The preparation of flavan-3-ol thioethers standards could be performed only starting from oligomers of known composition (also not easily available) and requires lengthy purification steps (Torres and Bobet, 2001). In the present study the retention times of the flavan-3-ol thioethers were identified by extemporary HPLC-MS measurements performed in an external laboratory using the same column and method and Agilent 1290 Infinity HPLC system equipped with a mass detector (Agilent 6130 quadrupole MS).
- Sample and standard preparation
- MALDI-TOF mass spectrometry
- Sample preparation
- Dissolve the dried plant extracts (2.5 mg) in 1 ml of aqueous acetone (50%) and sonicate (35 kHz, 250 W) at room temperature for 30 min.
- Prepare a matrix solution by dissolving 10 mg of 2,5-dihydroxybenzoic acid in 1 ml of pure acetone.
- In Eppendorf tube (1 ml) mix 10 µl of the extract solution with 10 µl of matrix solution and spike it with 1 µl of KCl (10 g/L in water) to enhance the formation of potassiated ions and suppress the formation of sodiated and/or other types of ions.
Note: Plant tissues might contain different kinds of salts, which may consequently contribute to the ion formation during the MALDI ionization process. For correct spectra interpretation it is therefore essentially important to ensure the formation of only one type of ions (e.g., either sodiated or potassiated, or others). Of particular concern are already mentioned sodium and potassium ions, because these two salts are common constituents in plant tissues. The mass difference between Na+ and K+ is 16 amu, which is also a mass difference between the building units of the condensed tannins (Table 4). Thus, unintended formation of Na+ and K+ ions at the same time may result in errors in spectra interpretation. - Deposit 5 µl of the mixture on the MALDI-TOF stainless steel plate and allow it to dry at room temperature for about 1 h.
- The samples should be stored at room temperature and measured at the same day of the preparation.
- Dissolve the dried plant extracts (2.5 mg) in 1 ml of aqueous acetone (50%) and sonicate (35 kHz, 250 W) at room temperature for 30 min.
- Measurement
- Set the MALDI TOF mass spectrometer in positive linear mode with a monitoring range between 700 and 4,500 m/z.
- Calibrate the MALDI TOF mass spectrometer with the calibration standard (e.g., Peptide calibration standard II, Bruker Daltonics, Germany)
- Perform the measurement on a MALDI-TOF mass spectrometer collecting about 700 laser shots for each sample.
- Set the MALDI TOF mass spectrometer in positive linear mode with a monitoring range between 700 and 4,500 m/z.
- Sample preparation
Data analysis
- HPLC-UV after thiolysis
- The molar concentration (µmol/L) of each flavan-3-ol monomer released from the condensed tannins (FTANN) is corrected according to the equation (1):
FTANN = Fth-FNth (1)
Where,
Fth = molar concentration (µmol/L) of the flavan-3-ol (or its thioether) in the thiolyzed sample,
FNth = molar concentration (µmol/L) of the flavan-3-ol (or its thioether) in the non-thiolyzed sample.
For the flavan-3-ol thioethers no correction is needed, because they are obviously not present as native compounds in the extracts.
In Table 2 an example of calculation based on the chromatogram of Figure 3 is reported.
Table 3. Data analysis from the HPLC-UV chromatogram of Figure 3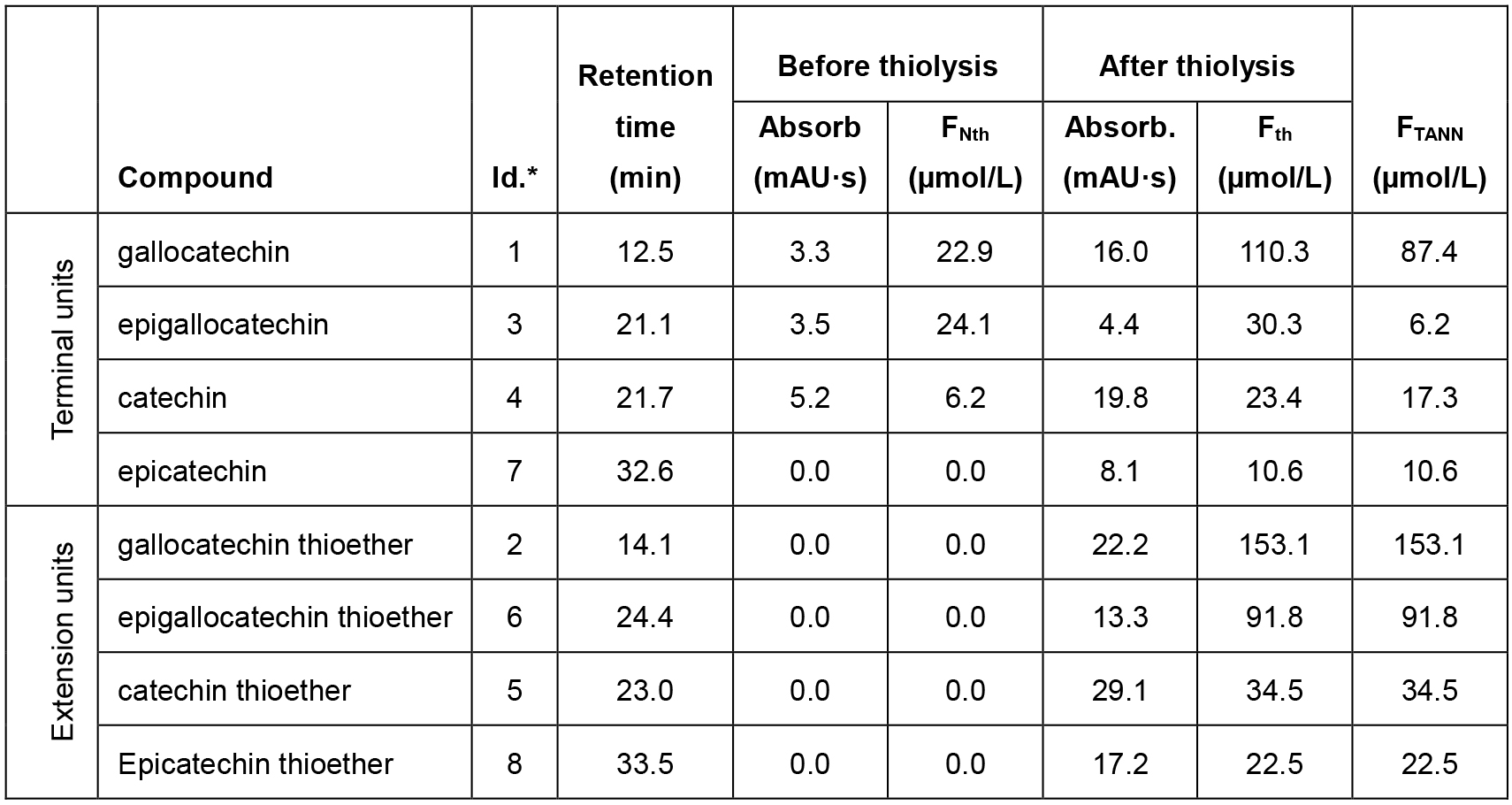
*Note: Identification label used in Figure 3. - The measured amounts of the released monomeric units provide the following information on the molecular structure of condensed tannins (Bianchi et al., 2015):
- The sum of the molar concentrations of a specific flavanol unit (both as terminal and extension unit) gives an indication about the amount of such monomeric unit in the tannin.
- The relative ratios between different monomeric units present in the tannin (also as stereoisomers like catechin and epicatechin) could be estimated by the relative ratios between the associated released flavanols.
- Differences between the configuration of terminal units and those of the extension units could be identified.
- The mean degree of polymerization (mDP) of the condensed tannins could be estimated with the equation (2):
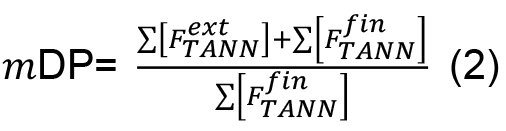
Where,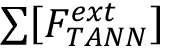 = molar concentration (µmol/L) of the total released flavan-3-ol extension units (flavan-3-ol thioethers) after thiolysis,
= molar concentration (µmol/L) of the total released flavan-3-ol extension units (flavan-3-ol thioethers) after thiolysis,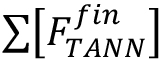 = molar concentration (µmol/L) of the total released flavan-3-ol terminal units (native flavan-3-ol) after thiolysis.
= molar concentration (µmol/L) of the total released flavan-3-ol terminal units (native flavan-3-ol) after thiolysis.
For the example in Figure 3 and using the data reported in Table 2, the calculation results as follow: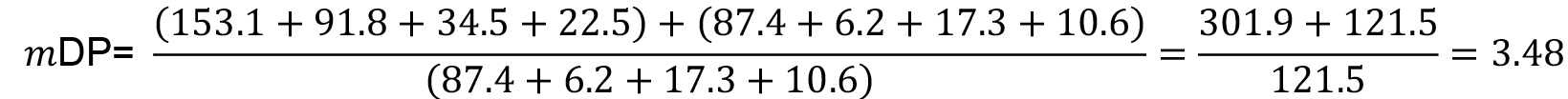
- The sum of the molar concentrations of a specific flavanol unit (both as terminal and extension unit) gives an indication about the amount of such monomeric unit in the tannin.
- The molar concentration (µmol/L) of each flavan-3-ol monomer released from the condensed tannins (FTANN) is corrected according to the equation (1):
- MALDI-TOF mass spectrometry
- Typical MALDI MS spectrum of plant extract containing condensed tannins is shown in Figure 4. The spectrum contains several groups of peak patterns regularly distanced from each other along the m/z axis. Each peak represents a tannin oligomeric chain with a defined composition of tannin monomeric units, which masses are reported in Table 4. The masses of the monomeric units are calculated from the masses of the monomers (Figure 1) by subtracting the mass of 2 hydrogen atoms (e.g., catechin monomeric unit = 290.3 - 2 = 288.3 Da). The distance between the repeating pattern of peaks (Figure 4) permits to identify the type of monomeric unit in the oligomer chain and the corresponding class of tannin (Table 4).
Note: MALDI-TOF analysis doesn’t permit to distinguish between monomeric units having the same mass (e.g., catechin and robinetinidol). - Within a peak group, peaks distanced by 16 m/z or multiple of 16 m/z (Figure 4) indicate the occurrence of various monomeric (building) units in the same condensed tannin, e.g., gallocatechin (304.3 Da) and catechin (288.3 Da).
Table 4. Typical monomeric units for different classes of condensed tannins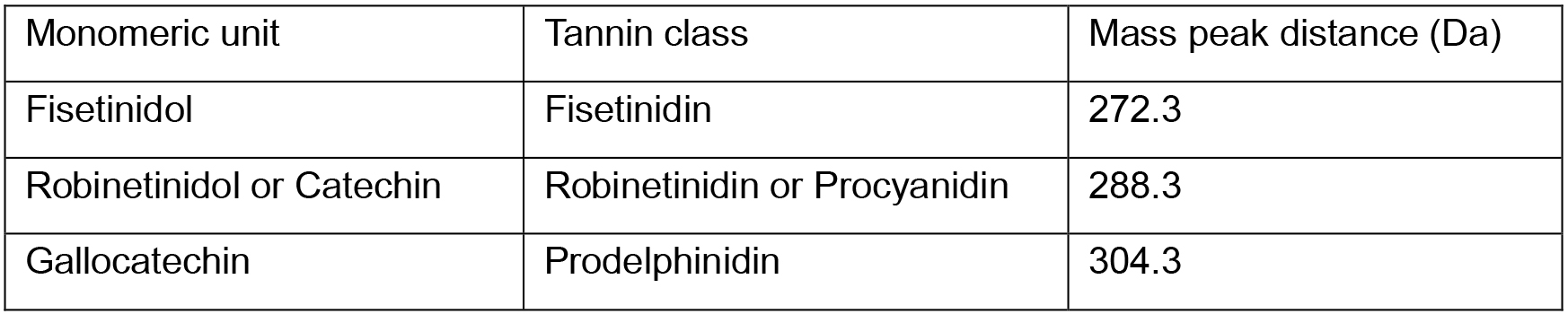
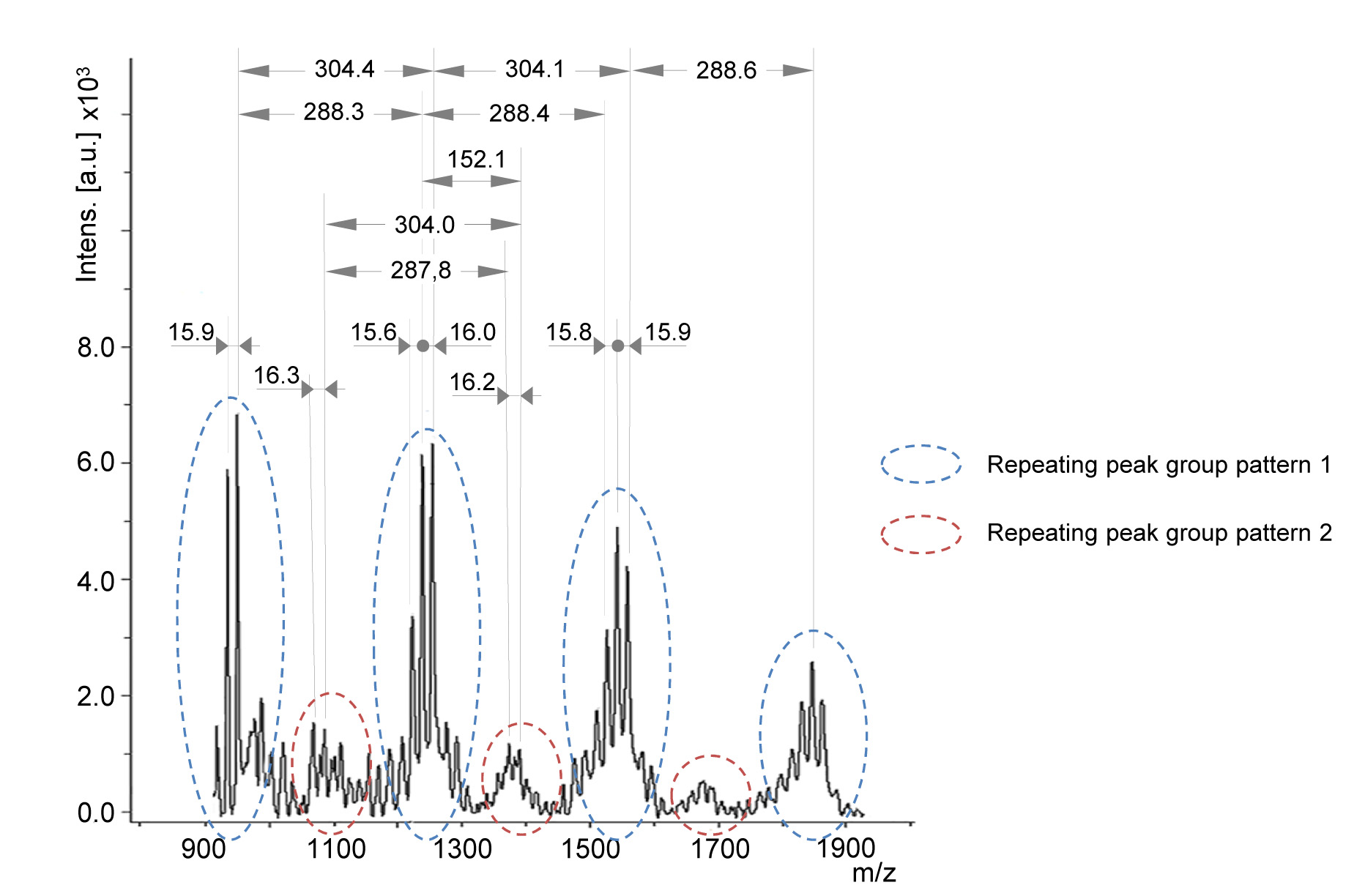
Figure 4. MALDI-TOF mass spectrum between 800 and 2,000 m/z of Silver fir (Abies alba [Mill.]) bark extract showing mass peaks associated to condensed tannins containing catechin (or robinetinidol), gallocatechin and gallate esters
- The identification of the monomeric unit composition in correspondence of a peak of the MALDI-TOF spectrum, can be performed using the equation (3):
M + Cat+ = x∙272.3 + y∙288.3 + z∙304.3 + MCat + 2.0 (3)
Where,
x, y and z are the number of fisetinidol, catechin (or robinetinidol) and gallocatechin units in the oligomeric chain, respectively,
M is m/z value of the oligomeric chain observed in the mass spectrum,
MCat is the mass of the cationization ion (e.g., Na+, K+),
2.0 is the weight of the two hydrogen atoms at the ends of the oligomeric chain.
In the presence of building units different than flavan-3-ols (e.g., stilbenes) or moieties (e.g., glycosides, gallic acid ester), additional repeating peak groups will be detectable in the mass spectrum, shifted from the non-esterified series by a gap corresponding to the mass of the non-flavanol unit or of the moiety (Bianchi et al., 2014). E.g., the presence of an oligomer series containing a gallate ester moiety (152.1 Da) results in peak groups distanced from the non-esterified series by 152 m/z, as reported in Figure 4. In this case the general Eq. 3 should be modified including the detected moiety, as shown in Eq. 4. In this case, for simplicity, the 272.3 unit representing fisetinidol was deleted (as it was not detected in the spectrum of Figure 4) and the mass of the cationization ion K+ (39.1) is explicitly reported. The variables y and z have the same meaning as in Eq. 3 and w is the number of gallate ester moieties in the tannin oligomer.
M + K+ = y∙288.3 + z∙304.3 + w∙152.1 + 39.1 + 2.0 (4)
The application of such equation is shown in Table 5.
Table 5. Interpretation of the MALDI-TOF spectrum reported in Figure 4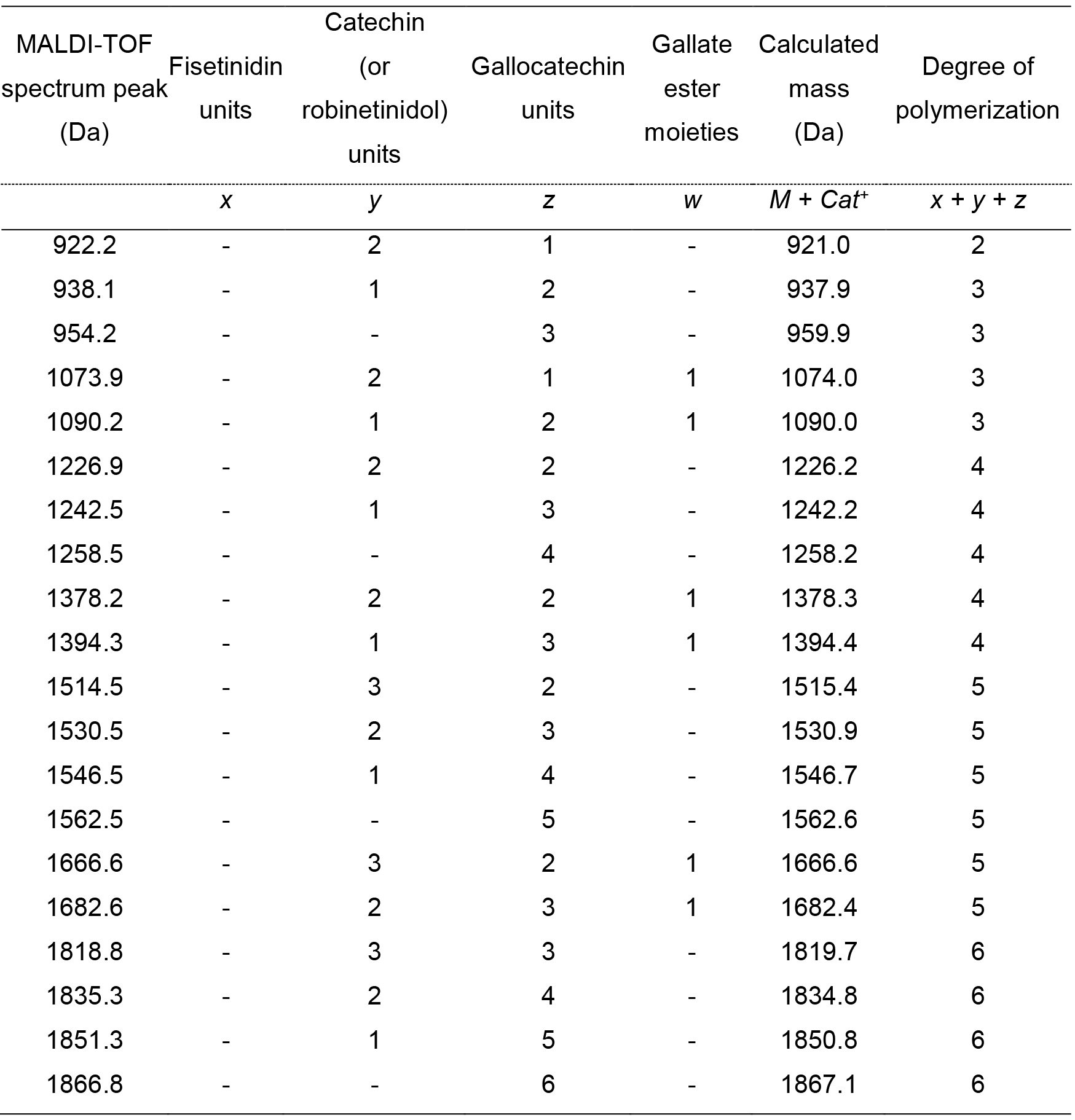
The degree of polymerization (DP) of each tannin oligomer is defined by equation (5):
DP = x + y + z (5)
Where,
x, y and z have the same meaning as in equation (3).
In presence of glycosil or ester moieties, their number should not be accounted in the calculation of the DP, because they are considered as an integrant part of the monomeric unit, e.g., flavanol glucosides, flavanol gallates (Bianchi et al., 2014; Bianchi et al., 2015).
- Typical MALDI MS spectrum of plant extract containing condensed tannins is shown in Figure 4. The spectrum contains several groups of peak patterns regularly distanced from each other along the m/z axis. Each peak represents a tannin oligomeric chain with a defined composition of tannin monomeric units, which masses are reported in Table 4. The masses of the monomeric units are calculated from the masses of the monomers (Figure 1) by subtracting the mass of 2 hydrogen atoms (e.g., catechin monomeric unit = 290.3 - 2 = 288.3 Da). The distance between the repeating pattern of peaks (Figure 4) permits to identify the type of monomeric unit in the oligomer chain and the corresponding class of tannin (Table 4).
- Concluding remarks
- Combination of MALDI-TOF mass spectrometry with HPLC-UV after acid thiolysis of hot water extracts from plant tissues permits to obtain various indicators on the condensed tannin structure present in the extracts.
- Both methods provide indication on the predominant monomeric unit, and therefore the class of the condensed tannins present in the extracts.
- HPLC-UV combined with thiolysis gives information on the mean degree of polymerization and could also distinguish between monomeric units with different stereochemistry. Its main limitation is the reduced thiolysis efficacy in case of side chain (branching) and the non-cleavability of interflavonyl bonds between monomeric units different than catechin, gallocatechin or their epimers.
- MALDI-TOF mass spectrometry provides information on the degree of polymerization, where obviously peaks with the highest detected m/z ratio indicate the maximal (observed) polymerization degree of the condensed tannin in the measured sample. Furthermore, the presence of monomeric units different than simple flavan-3-ols could also be elucidated by the MALDI-TOF mass spectra.
- Combination of MALDI-TOF mass spectrometry with HPLC-UV after acid thiolysis of hot water extracts from plant tissues permits to obtain various indicators on the condensed tannin structure present in the extracts.
Recipes
- Thiolysis media
500 mg cysteamine hydrochloride HPLC grade
0.2 ml hydrochloric acid 37% HPLC grade
9.3 ml methanol HPLC grade
Preparation of thiolysis media: - Weigh 500 mg of cysteamine hydrochloride on an analytical balance in a 20 ml glass flask.
- Add 9.3 ml of methanol with a calibrated Eppendorf pipette and shake the mixture until complete dissolving of any solid residual.
- Add 0.2 ml of concentrated HCl (37%) with a calibrated Eppendorf pipette and sonicate the mixture (35 kHz, 250 W) at room temperature for 5 min.
- Store the prepared thiolysis media in refrigerator (3-8 °C) protecting from light and air until use.
- HPLC mobile phases
- Solution A: 0.10% v/v trifluoracetic acid in water (about 55 ml/sample)
- Solution B: 0.08% v/v TFA in acetonitrile water mixture (ACN:Water = 4:1, v/v) (about 25 ml/sample)
Acknowledgments
We thank the Swiss National Research Program 'Resource Wood' (NRP66) for the financial support of this work. This protocol was developed starting from the procedures originally described in Matthews et al. (1997), Torres and Lozano (2001), Pasch et al. (2001) and Monagas et al. (2010).
References
- Bianchi, S., Gloss, A., Kroslakova, I., Mayer, I., Pichelin, F. (2014). Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry. Ind. Crop Prod 61: 430-437.
- Bianchi, S., Kroslakova, I., Janzon, R., Mayer, I., Saake, B. and Pichelin, F. (2015). Characterization of condensed tannins and carbohydrates in hot water bark extracts of European softwood species. Phytochemistry 120: 53-61.
- Cheynier, V., Labarbe, B., Moutounet, M. (2001). Estimation of procyandin chain lenght. Method in Enzymology 335, 82-94.
- Garnier, S., Pizzi, A., Vorster, O.C., Halasz, L. (2001). Comparative rheological characteristics of industrial polyflavonoid tannin extracts. J Appl Poly Sci 81: 1634-1642.
- Hümmer, W. and Schreier, P. (2008). Analysis of proanthocyanidins. Mol Nutr Food Res 52(12): 1381-1398.
- Jerez, M., Selga, A., Sineiro, J., Torres, J.L. and Núñez, M.J. (2007). A comparison between bark extracts from Pinus pinaster and Pinus radiata: Antioxidant activity and procyanidins composition. Food Chem 100(2): 439-444.
- Matthews, S., Mila, I., Scalbert, A., Pollet, B., Lapierre, C., Hervé du Penthoat, C.L.M., Rolando, C., Donnelly, D.M.X. (1997). Method for estimation of proanthocyanidins based on their acid depolymerization in the presence of nucleophiles. J Agric Food Chem 45(4): 1195-1201.
- Monagas, M., Quintanilla-Lopez, J.E., Gomez-Cordoves, C., Bartolomé, B., Lebron-Aguilar, R. (2010). MALDI-TOF MS analysis of plant proanthocyanidins. J Pharm Biomed Anal 51(2): 358-372.
- Pasch, H., Pizzi, A., Rode, K. (2001). MALDI-TOF mass spectrometry of polyflavonoid tannins. Polymer 42(18): 7531-7539.
- Pizzi, A., Stephanou, A. (1994). Fast vs. slow-reacting non-modified tannin extracts for exterior particleboard adhesives. Holz als Roh- und Werkstoff 52(4): 218-222.
- Torres, J. L. and Bobet, R. (2001). New flavanol derivatives from grape (Vitis vinifera) byproducts. Antioxidant aminoethylthio-flavan-3-ol conjugates from a polymeric waste fraction used as a source of flavanols. J Agric Food Chem 49(10): 4627-4634.
- Torres, J. L., Lozano, C. (2001). Chromatographic characterization of proanthocyandins after thiolysis with cysteamine. Chromatographia 54(7): 523-526.
- Yoneda, S., Nakatsubo, F. (1998). Effects of the hydroxylation patterns and degrees of polymerization of condensed tannins on their metal-chelating capacity. J Wood Chem Technol 18(2): 193-205.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bianchi, S., Kroslakova, I. and Mayer, I. (2016). Determination of Molecular Structures of Condensed Tannins from Plant Tissues Using HPLC-UV Combined with Thiolysis and MALDI-TOF Mass Spectrometry. Bio-protocol 6(20): e1975. DOI: 10.21769/BioProtoc.1975.
Category
Plant Science > Plant biochemistry > Other compound
Plant Science > Plant physiology > Plant growth
Biochemistry > Other compound > Tannin
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











