- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A 3D Culture System of Human Immortalized Myometrial Cells
Published: Vol 6, Iss 20, Oct 20, 2016 DOI: 10.21769/BioProtoc.1970 Views: 9201
Reviewed by: Andrea IntroiniJalaj GuptaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3788 Views

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1255 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 144 Views
Abstract
Myometrium forms the middle layer of the uterus and is mainly composed of the smooth muscle cells. The cells in vitro are usually grown in a single layer (2-dimensional; 2D) format, whereas in vivo cells are structured in an extracellular matrix scaffolding that allows the cells to communicate and respond to environmental cues. We have developed human myometrium and leiomyoma 3-dimensional (3D) culture, wherein the cells retain their molecular characteristics and respond to environmental cues (Malik and Catherino, 2012; Malik et al., 2014).
Keywords: MyometriumBackground
In the last decade a certain shift is observed as more laboratories move from using the artificial 2D format of cell culture into 3D cell culture model system, where the cells are grown in a matrix that allows them to attach and attain a more physiologic configuration. This model system provides the cells with a more natural state of differentiation and the cultured cells develop an in vivo tissue-like environment. This is a detailed protocol for myometrium 3D cell culture growth in a collagen-I matrix, modified from Malik and Catherino (2012).
Materials and Reagents
- 8 chamber glass slides (8 well culture slides) (Corning, Falcon®, catalog number: 354108 )
- 5 ml, 10 ml and 25 ml serological pipettes, sterile, individually wrapped (Thermo Fisher Scientific)
- 15 ml and 50 ml conical sterile polypropylene centrifuge tubes (Thermo Fisher Scientific)
- Microscope slides (Thermo Fisher Scientific, Fisher Scientific, catalog number: 12-5446 )
- Coverslips (22 x 60 mm) (Thermo Fisher Scientific, Fisher Scientific, catalog number: 12-544G )
- 6-well culture plate
- 12-well cell culture plate
- T75 flask
- Aerosol barrier pipette tips (10 µl to 1,000 µl) (Thermo Fisher Scientific)
- Unfiltered 1 ml pipette tips (Thermo Fisher Scientific, Fisher Scientific, catalog number: 13-611-101 )
- 0.45 µm millex syringe filter unit (EMD Millipore, catalog number: SLHA02510 )
- Millex vented 0.22 µm syringe filter unit (EMD Millipore, catalog number: SLGSV255F )
- Kimberly-ClarkTM Kimwipes (Thermo Fisher Scientific, Fisher Scientific, catalog number: 06-666A )
- Myometrial cells
- Rat tail collagen-I
*Cultrex® 3-D culture matrix rat collagen I: 4 mg/ml (Trevigen, Cultrex®, catalog number: 3447-020-01 )
Collagen type I: 4.48 mg/ml (EMD Millipore, catalog number: 08-115 )
Collagen I, high concentration: 8-11 mg/ml (Corning, catalog number: 354249 )
*Note: Rat tail collagen-I from Trevigen is the most commonly used matrix in the lab but depending on the concentration of the gel to be made, we routinely use collagen-I from other vendors as listed. Follow the manufacturer’s conditions on storage as improper storage can lead to increased viscosity of the collagen and difficult to handle. - 10x PBS (filtered through 0.2 µm filter) (Thermo Fisher Scientific, GibcoTM, catalog number: 70011044 )
- Sodium hydroxide (NaOH, 6 N) (VWR, catalog number: JT5672-2 )
- Double distilled water (DD water; Filtered through 0.2 µm filter)
- Trypsin-EDTA (0.05%) (Thermo Fisher Scientific, GibcoTM, catalog number: 25300054 )
- Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12, with phenol red) (Thermo Fisher Scientific, GibcoTM, catalog number: 11320-033 )
- Fetal bovine serum (FBS) (Defined) (GE Healthcare, HycloneTM, catalog number: SH30070.03 )
- Glutamax (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- Penicillin-streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Fungizone (Thermo Fisher Scientific, GibcoTM, catalog number: 15290018 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: P6148 )
Or 16% paraformaldehyde solution (Electron Microscopy Sciences, catalog number: 15710 ) - Glycine (Sigma-Aldrich, catalog number: G5417-100G )
- Triton X-100 (Sigma-Aldrich, catalog number: 93443 )
- Normal goat serum (NGS) (Abcam, catalog number: ab156046 ) (Dilution in 1x PBS)
- Secondary antibody: Alexa 488 (follow manufacturers dilution instructions) (Thermo Fisher Scientific, InvitrogenTM, catalog number: A11008 )
- Primary antibody to smooth muscle alpha actin (0.2 mg/ml) (Abcam, catalog number: ab5694 )
- Prolong® Gold antifade mountant with DAPI (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: P36931 )
- DPBS (no magnesium or calcium) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190144 )
- Bovine serum albumin (BSA) (35% solution in DPBS) (Sigma-Aldrich, catalog number: A7979 ), Dilution in 1x PBS
- 10% growth media (see Recipes)
- 5% growth media (see Recipes)
- 1 N NaOH (see Recipes)
- 3 mg/ml collagen gels (see Recipes)
- 0.15 M glycine/PBS (see Recipes)
- 4% paraformaldehyde/PBS (see Recipes)
- Blocking buffer in PBS (see Recipes)
- Primary ab dilution buffer in PBS (see Recipes)
Notes:
a.Keep #1-3 at -20 °C if possible or 4 °C overnight (I have a small -20 °C freezer right next to my culture hood).
b.Keep #15-18 on ice before the start of experiment.
Equipment
- Microscope (Nikon, model: Eclipse TS100 )
- Centrifuge (Eppendorf, model: 5804R )
- Automated cell counter (Bio-Rad Laboratories, model: TC 20TM )
- Hemocytometer (if automated cell counter not available)
- Metal 50ml holder (e.g., Blue anodized aluminum [Thomas Scientific, catalog number: 1225W71 ])
Note: In -20 °C if possible or 4 °C overnight. - 95% air/5% CO2 incubator at 37 °C and 95% relative humidity
- Shaker at 4 °C and one at room temperature
- Vacuum aspirator
- Hot plate/stirrer (Fisher Scientific)
- Pyrex beaker (100 ml) (Fisher Scientific)
- Flat tip forceps (Thermo Fisher Scientific, Fisher Scientific, catalog number: 16-100-111 )
- Microscope: confocal laser microscope: (e.g., Carl Zeiss, model: ZEISS LSM 800 )
Procedure
Note: All steps to be carried out under cell culture hood, i.e., sterile conditions.
- Determination of required volumes
Determine the volume of collagen, neutralizing solution, 10x PBS and growth media needed as follows:- Calculate the volume of collagen needed to make a collagen gel of 1.3-3 mg/ml final concentration for 8-chambered slide (0.5 ml/well) or 6-well culture plate (2 ml/well).
Note: We typically make extra gel solution (~1 ml extra) to account for the viscosity of collagen, and to ensure we have enough for each gel. - The amount of neutralizing solution (freshly made 1 N NaOH) is calculated as 0.023x volume of collagen.
- 10x PBS is added as 1x (v/v for total volume). The remaining volume is made up with filtered DD water.
Note: If not using 10x PBS then replace the final volume with 5% growth media.
- Calculate the volume of collagen needed to make a collagen gel of 1.3-3 mg/ml final concentration for 8-chambered slide (0.5 ml/well) or 6-well culture plate (2 ml/well).
- Preparation of cells
- Myometrial cells (immortalized cells; see Malik et al., 2008) plated in T75 flask should be 50%-80% confluent before trypsinization.
Note: The cells should be actively dividing and not contact inhibited as would be the case if 100% confluent. - Completely remove the growth media from the flask before adding 3 ml of trypsin. Make sure whole surface area of the T75 flask is covered by swirling the trypsin solution around. Leave for 3 min, tap the flask and observe under the microscope to see if myometrial cells are detached.
- Once the cells are observed to have detached from surface of the flask, stop the trypsinization reaction by adding growth media (10%) at 1:1 (v/v) ratio. Pipette the cells in 50 ml conical tube.
Note: The growth media 10% DMEM/F12 is at room temperature. - Centrifuge the cells at 800 rpm/75 x g for 10 min at 25 °C.
- Resuspend the pellet in smallest volume of 10% growth medium. *It is very important at this point to get the myometrial cells into single cell suspension.
Note: Resuspension volume depends on how many cells you have, e.g., 1 x 106 cells can be resuspended in 0.75-1 ml growth media. *If unable to get into single cell suspension, more 10% growth media can be added but make a note of total volume. The speed of mixing the cells should be slow and gentle as high speed can lead to shearing of cells. - Count cells using automated cell counter or hemocytometer. Plan to use 1-1.5 x 105 cells/ml of 3D collagen gel to give a final cell count of ~5 x 104 cells/well in 8 chambered slide.
Note: Number of cells to be added should be optimized based on rate of division of the cells being used. Cancer cells tend to divide faster compared to normal cells. - The cells can be kept under the hood (under sterile conditions) for up to 15 min. As the cells tend to settle to the bottom of the tube on long storage, gentle mixing is recommended before addition to the prepared collagen matrix.
- Myometrial cells (immortalized cells; see Malik et al., 2008) plated in T75 flask should be 50%-80% confluent before trypsinization.
- Culture of myometrium cells in 3D culture
Note: The following steps are critical.- Before the start of experiment all solutions (see Materials and Reagents) should be on ice and ready. Metal holder for conical tubes, 50 ml conical tubes and the serological pipettes in -20 °C or 4 °C.
- As the myometrium cells are being counted, all solutions on ice including the rat tail collagen should be moved under the cell culture hood.
- Once the cells are counted and ready, the following steps should be accomplished within a 10 min time period to avoid loss of cell viability.
- Have a 50 ml conical tube in cold metal holder ready in the cell culture hood.
- To create the collagen gels, add the appropriate volume of 10x PBS, DD water (or 5% growth media) to the cold 50 ml tube. Next, add the neutralizing solution, and finally add the collagen. Mix all solutions together using cold pipettes.
Note: The viscous collagen tends to move smoothly when using cold pipettes as compared to ones at room temperature. Make sure to keep the neutralizing solution and collagen cold (on ice) until you are ready to use them, as higher temperatures increase the rate of collagen polymerization. If 5% media is used, the solution will have yellow/pinkish hue. - Add the cells (0.5 ml, equivalent to 5 x 105 cells) using a 1 ml Eppendorf pipette in a swirling motion in the middle of the collagen solution in the cold 50 ml tube. Quickly mix the solution again with a cold pipette.
Important note: From this step onward there should be no stopping time. The pipetting steps should be carried out quickly as the cells are in cold solution they can lose viability. - Pipette 0.5 ml per well in an 8-chambered slide. Take a sterile Eppendorf pipette tip and run it along the inside wall of the wells. This tends to evenly spread the collagen solution such that the gel does not have a concave structure due to surface tension.
Note: The chambered slides are also kept cold. They can be placed on an ice pack inside the culture hood. - Place the lid back on the chamber slide and immediately place the covered slides in the incubator, at 37 °C. The collagen gel with cells will polymerize in approximately 30 min.
- Add 0.35 ml of 5% growth medium gently to the polymerized 3D culture. The volume should cover the gel completely. Cell cultures are maintained in a humidified 95% air/5% CO2 incubator at 37 °C. *Fresh media is replaced every day or as required.
Note: As the myometrial cells (smooth muscle cells) grow, the collagen matrix tends to contract. Addition of 5% media instead of 10% media slows down this process.
*The cell culture media DMEM/F12 contains phenol red which is a pH indicator. When media in 3D myometrium cell culture changes from red to yellow, it indicates to acidic pH of the media. This is indicative of actively dividing cells. To maintain a near neutral pH (7.2-7.3), it is important to replace media of the 3D cultures in the 8-chamber slides every day. - Microscope image: When observed under the microscope the morphology of myometrial cells, added into the 3D collagen gel, includes spherical shape and translucent appearance, indicating that the cells are healthy and alive. As the cells from human myometrium are smooth muscle cells, within 8-10 h our cells obtain the long elongated spindle shape characteristic of smooth muscle cells. Within 2 weeks the cells form a network of spindle shaped cells (see Figure 1).
Important note: If the myometrium cells have not obtained spindle shape within 48 h of plating in collagen 3D culture it may be assumed that the cells are no longer viable.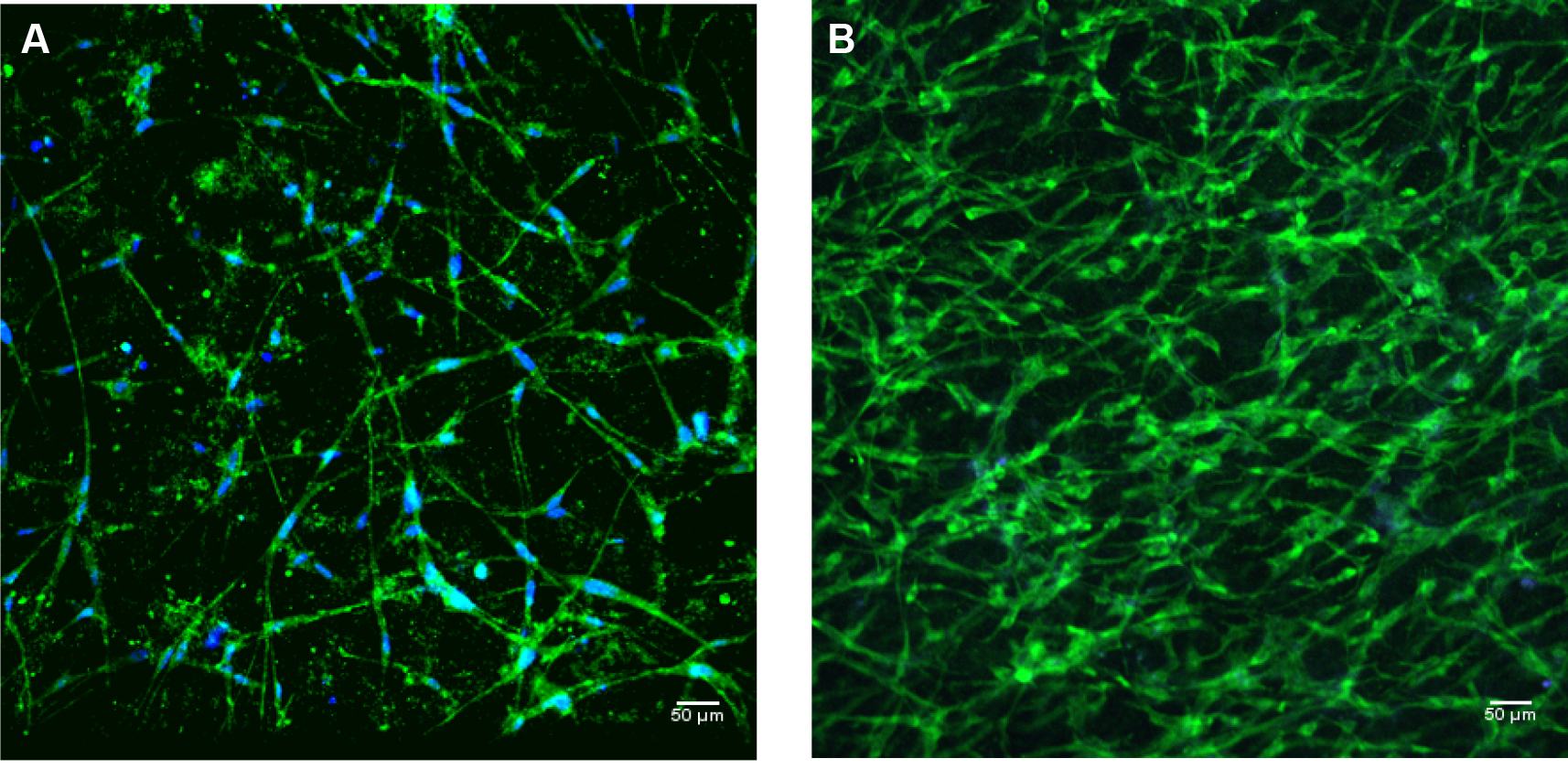
Figure 1. 3D myometrial culture whole gel mount exposed to anti-alpha smooth muscle actin (intercellular protein, green); and DAPI (blue) for nuclear identification. A. 3D culture after 1-1.5 weeks of growth; B. 3D myometrial culture after 4 weeks of growth.
- Before the start of experiment all solutions (see Materials and Reagents) should be on ice and ready. Metal holder for conical tubes, 50 ml conical tubes and the serological pipettes in -20 °C or 4 °C.
- Cytoimmunofluorescence of 3D cultures
The cytoimmunofluorescence labeling method was modified from Wozniak and Keely (2005) and Malik and Catherino (2012).- The 3D cultures were collected after approximately 4 weeks of growth or when a network of cells have formed (see Figure 2 for detailed cell percentage picture).
Note: A network of cells should have formed by 4 weeks of growth. This is assuming that all cells plated were viable.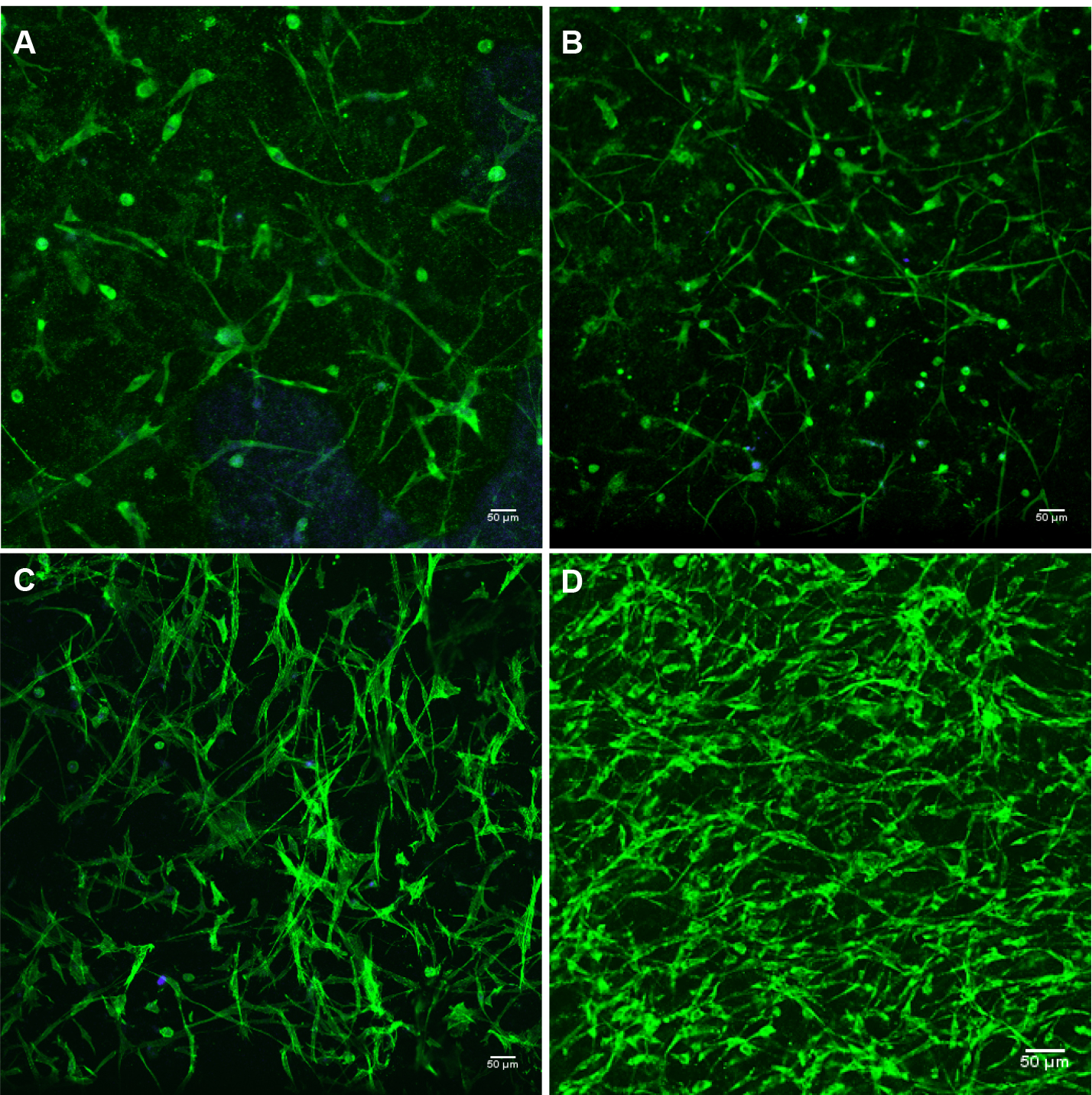
Figure 2. 3D myometrial culture whole gel mount exposed to anti-alpha smooth muscle actin (intercellular protein, green); and DAPI (blue) for nuclear identification. A. 10% confluent; B. 30% confluent; C. 50% confluent (2 weeks of growth); D. 80% confluent (4 weeks of growth). Confluence calculation is based on visual approximation. 10x magnification. - Fixation: After two 1 min washes with 1x PBS (without Mg2+ or Ca2+) the gels are fixed overnight in freshly made 4% paraformaldehyde, at 4 °C with gently shaking.
Note: Most washes are done in the chamber itself. Make sure the gels are completely covered. In an 8-chamber slide, 350-400 µl volume is required. The aspiration rate on the vacuum aspirator (same as in the culture hood) is kept to low. We usually attach an unfiltered 1 ml pipette tip with narrow tip to the aspirator tube. The aspiration should be done carefully as the collagen gels, if not firm, can get aspirated. - The gels are allowed to stabilize at room temperature (RT, 10 min) before a two 10 min wash with 1x PBS with gentle shaking at RT.
- The gels are incubated for 10 min in 0.15 M glycine. Followed by a 10 min wash of the gels with 1x PBS with gentle shaking.
Note: This step quenches any auto-fluorescence and/or fluorescence due to formaldehyde. - Permeabilization: The 3D gels are exposed to 0.05% Triton X-100 for 20 min at RT with gentle shaking, allowing permeabilization of the cells. Permeabilization is followed by two 10 min washes with 1x PBS with gentle shaking.
Note: The gels should be completely submerged in the solutions. In an 8-chamber slide, 350-400 µl volume is required, or as observed. - Blocking: The cells were blocked for 1 h at RT (or 30 min in 37 °C incubator). 350 μl of blocking buffer (see Recipes) is added to the chamber with 3D gel. If blocking at 37 °C the chamber slide should be kept in a moist chamber. Shaking is optional.
Note: For blocking step use normal serum of the animal in which the secondary antibody is made. We use NGS (normal goat serum) as our secondary antibodies are made in goat. Moist chamber: we use wet paper towels in a Petri plate and place the chamber slide on it. - If blocking was carried out at 37 °C, let the chamber slide cool down to room temperature (5 min at RT). Blocking buffer should be removed before incubation with primary antibody.
- Primary antibody: The gels are exposed to smooth muscle specific (SMC) α-actin antibody at 1:100 dilution (final concentration of 0.02 µg/ml). The primary antibody is made in dilution buffer (see Recipes) and gels are incubated for 2 h at RT or overnight at 4 °C in a moist chamber. Gentle shaking is optional.
Note: The primary diluting buffer is very similar to blocking buffer hence no wash in between. The gels should be completely submerged in buffer containing primary antibody. - Wash: Three washes of 10 min each in 1x PBS with gentle shaking.
Note: All steps after this wash are done under low light conditions. - Secondary antibody: The gels are incubated in Alexa 488 conjugated secondary antibody at a concentration of 1 µg/ml dilution buffer, for 40 min at RT. With gentle shaking.
Note: The secondary antibody is diluted and added to the chambers under low light conditions. - The gels are washed twice in 1x PBS for 10 min each with gentle shaking.
- Individual gels are removed from the chamber and placed on slides. Take a fresh slide and use flat tipped forceps to gently remove the gel from the chamber. Use one gel per slide. Discard the chamber slide as it is no longer sterile.
Note: As the collagen gels contract due to growth and contractile nature of the myometrium cells (smooth muscle cells), the gel size may reduce from a height of 5 mm to < 2 mm after the end of 4 weeks of growth (Figures 3A and 3B).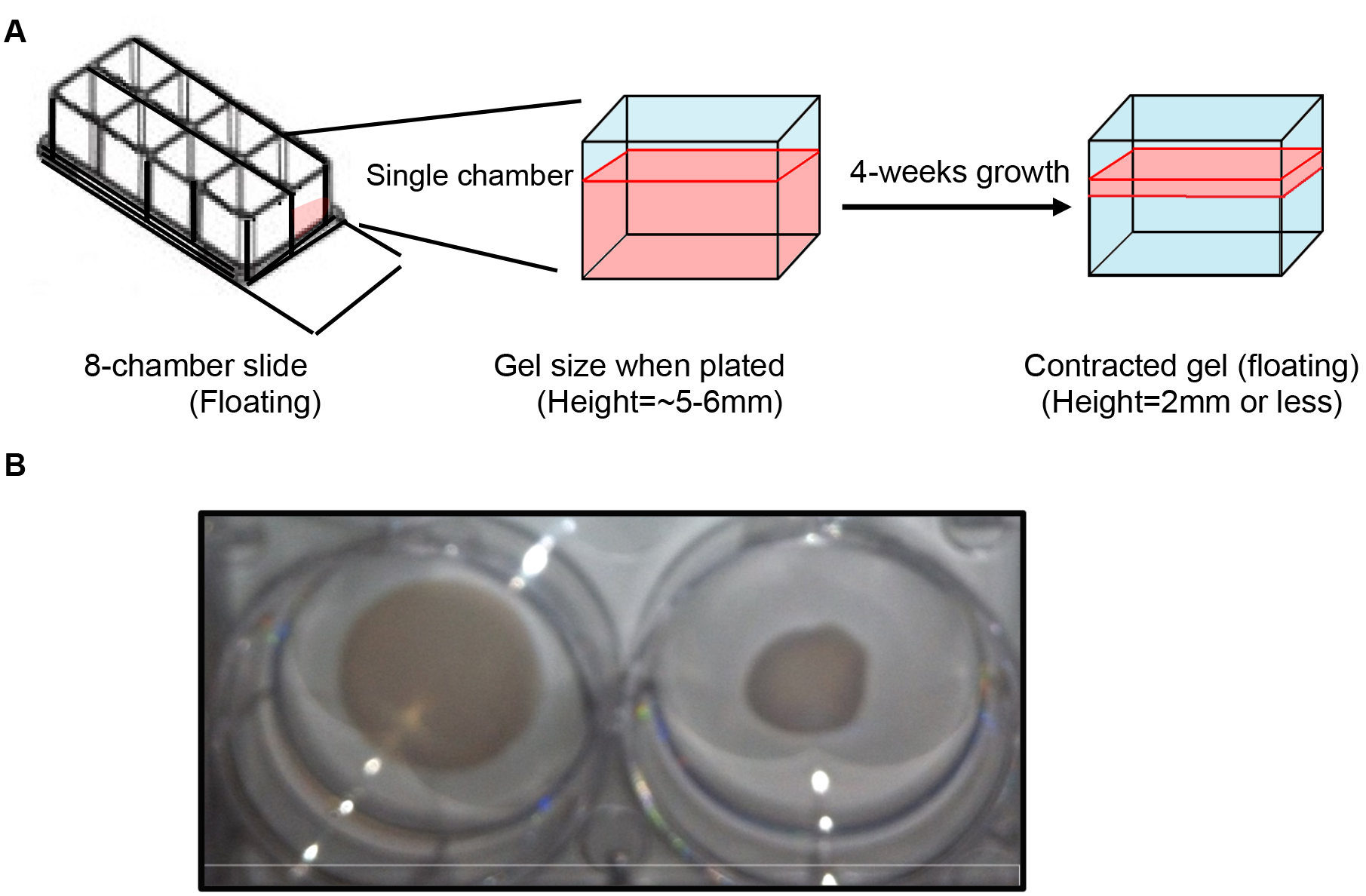
Figure 3. Contraction of 3D matrix due to myometrial cell growth. A. Depiction of myometrial cell 3D culture in a single chamber of 8-chamber slide. The area in red represents the collagen gel containing myometrial cells. After 4 weeks of growth the gel contracts to one third or less of original gel. B. Myometrial cell 3D culture grown in 12-well cell culture plate demonstrate a difference in overall size in 1 week old 3D culture compared to 90-100% confluent 5-week old 3D culture on the right. The cultures were transferred to 6-well cell culture plate for better visualization of the difference in size. - Extra solution is wicked using lint-free Kimwipes.
Note: The gel should not be too wet. If Kimwipe is gently touched to the corner of the gel it tends to soak up and remove all the extra solution. The gel should not be placed on the Kimwipe as it dries up quickly and it becomes difficult to separate the gel from the wipe. - Whole gel mount: One to two drops of Prolong Gold mounting media with DAPI is added on the gel. The gels are pressed down gently using a coverslip. Extra gel mount is wiped off and the slide allowed to dry overnight.
- Images are collected using the laser scanning confocal microscope.
- The 3D cultures were collected after approximately 4 weeks of growth or when a network of cells have formed (see Figure 2 for detailed cell percentage picture).
Notes
- If myometrium cells in 3D cultures are to be treated with any experimental drugs or compounds, the treatment should be carried out when the 3D cultures are 30-50% confluent (see Figure 2). Higher confluence leads toward contraction of gels.
- Gels with higher collagen concentration (4-6 mg) do not contract as quickly, due to increased stiffness. Stiffer collagen gels cannot undergo whole gel mount. Sections can be made with sharp blade and they can be mounted with Prolong Gold and analyzed.
- The whole gels (3-6 mg collagen) can be used for frozen section (cryosection) procedure. The gel is embedded in OCT, frozen and sectioned. The sections can be used for cyto-immunochemical or cyto-immunofluorescence analysis.
- The gels can also be fixed in formaldehyde (10%) overnight with shaking and paraffin embedded (Malik et al., 2016; Patel et al., 2016), for immunohistochemical analysis.
Recipes
- 10% growth media
Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12 with phenol red) containing 10% FBS defined, 1x glutamax, 1x antibiotics (Penicillin-streptomycin) and 1x fungizone. - 5% growth media
Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12 with phenol red) containing 5% FBS defined, 1x glutamax, 1x antibiotics (Penicillin-streptomycin) and 1x fungizone. - 1 N NaOH
1 ml of 6 N NaOH
5 ml DD water
Note: Neutralizing solution; freshly prepared and filtered through 0.2 µm filter. The neutralizing solution will control the pH of the final collagen gel. - 3 mg/ml collagen gels
3.0 ml of 5 mg/ml rat tail collagen-I
0.069 ml of 1 N NaOH
0.5 ml of 10x PBS
0.5 ml cells in growth media
0.931 ml DD water
Total volume: 5 ml
Note: 0.5 ml of 10x PBS and 0.931 ml DD water can be replaced by 1.431 ml of 5% growth media. - 0.15 M glycine/PBS
Molecular weight: 75.07
Weigh 563 mg and dissolve in 50 ml of 1x PBS.
Prepared solution can be stored at 4 °C. - 4% paraformaldehyde/PBS
Notes: All work to be done under a fume hood and with gloves. We always use freshly prepared solution. - Place 45 ml of DD water in a beaker and heat to 60 °C using a hot plate and with stirring.
- While stirring, add 2 g of paraformaldehyde powder slowly. Do not heat solution above 70 °C as paraformaldehyde tends to depolymerize.
- Add 1 drop of 1 N NaOH. The solution should clear within a couple of minutes. There will be some fine particles that will not go away. These will be removed by filtration later.
- Remove the beaker from heat and add 5 ml of 10x PBS. Mix well. Final volume will be 50 ml.
- Let it cool to room temperature. Filter through a 0.45 µM membrane filter and use immediately.
- *For dilution of 16% paraformaldehyde use 10 ml of the solution from 1 glass vial and add 30 ml of 1x PBS to give a final 40 ml solution of 4% paraformaldehyde in PBS.
- Blocking buffer in PBS
1% normal goat serum (NGS)
1% bovine serum albumin (BSA) - Primary ab dilution buffer in PBS
1% normal goat serum (NGS)
Acknowledgments
This protocol is a detailed version of the one published in Malik and Catherino (2012)
References
- Malik, M., Webb, J. and Catherino, W. H. (2008). Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol (Oxf) 69(3): 462-470.
- Malik, M. and Catherino, W. H. (2012). Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril 97(6): 1287-1293.
- Malik, M., Britten, J., Segars, J. and Catherino, W. H. (2014). Leiomyoma cells in 3-dimensional cultures demonstrate an attenuated response to fasudil, a rho-kinase inhibitor, when compared to 2-dimensional cultures. Reprod Sci 21(9): 1126-1138.
- Malik, M., Britten, J., Cox, J., Patel, A. and Catherino, W. H. (2016). Gonadotropin-releasing hormone analogues inhibit leiomyoma extracellular matrix despite presence of gonadal hormones. Fertil Steril 105(1): 214-224.
- Patel, A., Malik, M., Britten, J., Cox, J. and Catherino, W. H. (2016). Mifepristone inhibits extracellular matrix formation in uterine leiomyoma. Fertil Steril 105(4): 1102-1110.
- Wozniak, M. A. and Keely, P. J. (2005). Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol Proced Online 7(1): 144-161.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Malik, M., Britten, J. and Catherino, W. H. (2016). A 3D Culture System of Human Immortalized Myometrial Cells. Bio-protocol 6(20): e1970. DOI: 10.21769/BioProtoc.1970.
Category
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










