- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Corneal Stroma Fibroblast Primary Cell Culture
Published: Vol 6, Iss 19, Oct 5, 2016 DOI: 10.21769/BioProtoc.1960 Views: 10053
Reviewed by: Jingli CaoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Expansion of Airway Basal Cells and Generation of Polarized Epithelium
Hannah Levardon [...] Hongmei Mou
Jun 5, 2018 14443 Views

A Full Good Manufacturing Practice–Compliant Protocol for Corneal Stromal Stem Cell Cultivation
Mithun Santra [...] Gary H.F. Yam
Sep 20, 2024 2158 Views

Primary Mouse Choroidal Endothelial Cell Culture
Qiuhua Yang [...] Yuqing Huo
Jun 20, 2025 2187 Views
Abstract
This protocol is developed for primary cell culture of cornea stromal keratocytes isolated from neonatal mouse eyeballs. It provides an optimal condition to isolate stromal keratocytes which maintain high viability for cell culture.
Keywords: MouseMaterials and Reagents
- 15 ml Falcon tube (Corning, Falcon®, catalog number: 352095 )
- Petri dish (Thermo Fisher Scientific, Fisher Scientific, catalog number: FB0875713 )
- NunclonTM tissue culture dish (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 172931 )
- New born mice (postnatal day 0)
- 70% sterile ethanol (prepared from ethanol 190 proof) (Decon Labs, catalog number: 2801 )
- Distilled water (Thermo Fisher Scientific, GibcoTM, catalog number: 15230162 )
- PBS without Ca2+ Mg2+ (Thermo Fisher Scientific, GibcoTM, catalog number: 20012050 )
- Trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200072 )
- DMEM (Thermo Fisher Scientific, GibcoTM, catalog number: 11995-065 )
- Fetal bovine serum (GE Healthcare, HycloneTM, catalog number: SH30396.03 )
- Penicillin-streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- DMSO (Sigma-Aldrich, catalog number: D2650 )
- Liquid nitrogen
- Dispase II (Roche Diagnostics, catalog number: 04942078001 )
- Sorbitol
- Collagenase (Type L) (Sigma-Aldrich, catalog number: C8170 ), sterile-filtered, dissolved in PBS and stored at -20 °C.
- Hyaluronidase
- Disapse solution (see Recipes)
- Digestion buffer (see Recipes)
Equipment
- CO2 cabinet
- Chemical hood
- Tissue culture hood
- Phase-contrast inverted microscope (Electron Microscopy Sciences)
- Spring scissor (Fine Science Tools, catalog number: 15009-08 )
- Forceps (Fine Science Tools, catalog number: 00108-11 )
- Benchtop centrifuge (Hettich Lab Technology, model: Rotina 380 )
- Pipettes
- Hemocytometer (Hausser Scientific)
- CO2 incubator
Procedure
Note: All materials used in this experiment must be sterile or autoclaved to prevent contamination.
- Isolation of mouse corneal stroma keratocytes
- Euthanize five newborn pups using CO2 cabinet in a chemical hood. Briefly rinse the bodies in 70% (v/v) ethanol and PBS successively. Then place the eyeballs into a Petri dish and transfer it to a tissue culture hood.
- Dissect out eyeballs from each pup. Carefully cut corneas along the sclera rim using a surgical scissor and place all corneas in 15 ml Falcon tube with 10 ml of dispase solution at 4 °C in a horizontal orientation (Video 1).
Video 1. Isolation of mouse corneas from newborn pups under a stereo microscope. Mouse corneas are carefully cut along the sclera rim using a surgical scissor. - Transfer the tissues together with the dispase solution into a Petri dish (Video 1).
- Peel off and collect the loosened corneal epithelial sheets with forceps which can be cultured separately (Video 2).
Video 2. Dissection of corneal stoma tissues from dispase-treated cornea. Under a stereo microscope, corneal epithelial sheets are gradually stripped off from dispase digested mouse corneas and then collect corneal stroma tissues for cell culture. - Rinse the tissues in 10 ml PBS for two times to wash away dispase. Transfer tissues to a new 15 ml tube with 2 ml digestion buffer and incubate the tube at 37 °C with shaking (125 rpm) for 30 min.
- Spin for 5 min at 300 x g in a centrifuge at room temperature; carefully decant and discard supernatant. Wash once with 5 ml PBS by mixing thoroughly and centrifuge as above, discarding the supernatant.
- Add 0.5 ml trypsin-EDTA, mix thoroughly, and incubate at 37 °C for 20 min. Then centrifuge and discard supernatant as above (step A6), resuspend pellet in 0.5 ml DMEM medium containing 10% fetal bovine serum. Using 1 ml pipette, pipet up and down several times to break up cell aggregates.
- Euthanize five newborn pups using CO2 cabinet in a chemical hood. Briefly rinse the bodies in 70% (v/v) ethanol and PBS successively. Then place the eyeballs into a Petri dish and transfer it to a tissue culture hood.
- Primary culture
- Plate the suspension from step A7 onto one 6 cm tissue culture dish, avoiding tissue mass.
- Add 3 ml DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and grow the cells for 48 h at 37 °C in a CO2 incubator with 5% CO2. Next, replace the old growth medium with 3 ml DMEM containing 10% FBS without antibiotics every 3 days. Within 2 weeks from the time of establishment of the culture, confluent monolayers will form, displaying the typical fibroblast morphology (Figure 1).
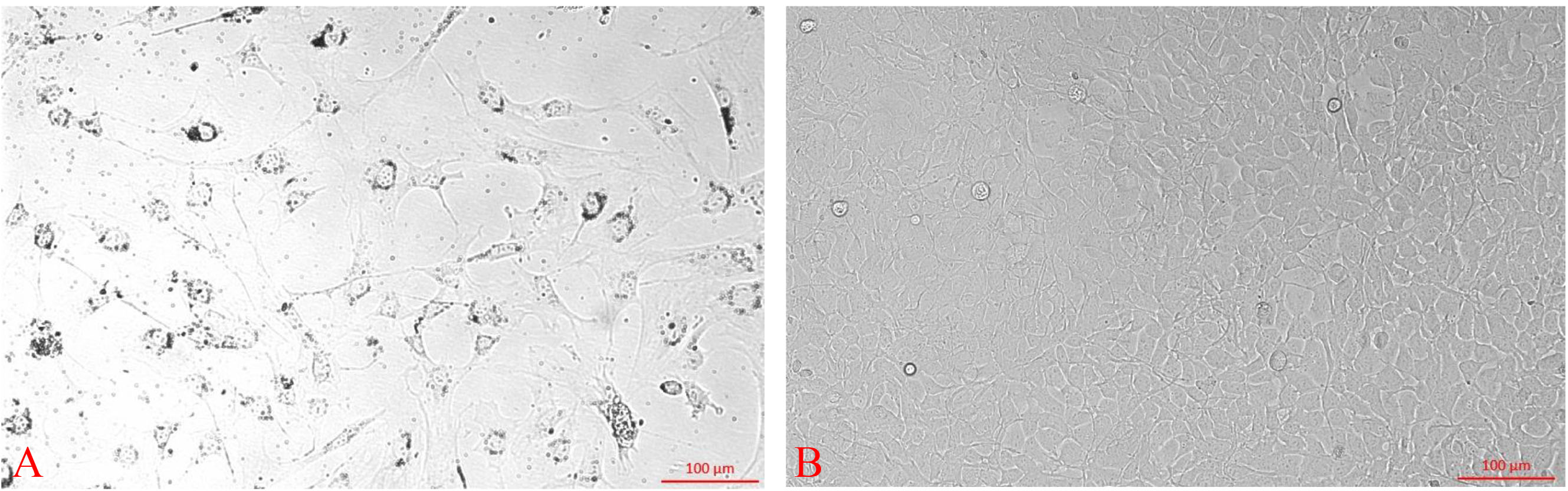
Figure 1. The morphology of mouse corneal fibroblast cultured for one (A) and two (B) weeks. Scale bar = 100 μm.
- Plate the suspension from step A7 onto one 6 cm tissue culture dish, avoiding tissue mass.
- Subculture
- At 80-90% confluency, aspirate the medium and rinse the cells with PBS. Add 0.5 ml trypsin-EDTA to trypsinize the cells in the culture plate and incubate it at 37 °C for 5 min to release cells from the culture plate. Next, add 5 ml DMEM + 10% FBS to stop the reaction.
- Collect the cells with medium into a 15 ml tube. Spin down the cells at slow speed (300 x g for 5 min). Discard the supernatant, resuspend the cells in DMEM culture medium and count the cells using a hemocytometer. Next, plate the cells at a density of 1 x 104 cells/cm2 onto a plate containing DMEM + 10% FBS medium without antibiotics. Incubate the culture in a CO2 incubator at 37 °C and 5% CO2.
- Change culture medium every 2-3 days and subculture the cells when 80-90% confluence.
- At 80-90% confluency, aspirate the medium and rinse the cells with PBS. Add 0.5 ml trypsin-EDTA to trypsinize the cells in the culture plate and incubate it at 37 °C for 5 min to release cells from the culture plate. Next, add 5 ml DMEM + 10% FBS to stop the reaction.
- Cryopreservation
Cryopreservation is necessary to maintain large quantities of cells from the same tissue samples for future experiments.- Release cells using trypsin-EDTA and centrifuge as for the subculture (step C1).
- Collect the cells with medium into a 15 ml tube. Spin down the cell at slow speed (300 x g for 5 min). Discard the supernatant, resuspend cells in DMEM culture medium and count the cells using a hemocytometer.
- Dispense aliquots of 2 x 106 cells/ml in culture medium with 10% DMSO.
- Store cells at -80 °C freezer for 24 h and then transfer cells to liquid nitrogen for long-term storage.
- To recover cells: thaw cells quickly in a 37 °C water bath and dilute cells tenfold with DMEM medium without antibiotics. Then subculture cells as above (step C).
- Release cells using trypsin-EDTA and centrifuge as for the subculture (step C1).
Recipes
- Dispase solution
Prepare 15 mg/ml dispase II, 100 mM sorbitol and 1% penicillin-streptomycin in DMEM basal medium.
Store at -20 °C. - Digestion buffer
Prepare 2.0 mg/ml collagenase and 0.5 mg/ml hyaluronidase in DMEM basal medium.
Store at -20 °C.
Acknowledgments
This work was supported by grant from the National Institute of Health/National Eye Institute (NIH/NEI) [RO1 EY21501, RO1 EY23086].
References
- Seluanov, A., Vaidya, A. and Gorbunova, V. (2010). Establishing primary adult fibroblast cultures from rodents. J Vis Exp (44).
- Zhang, Y., Yeh, L. K., Zhang, S., Call, M., Yuan, Y., Yasunaga, M., Kao, W. W. and Liu, C. Y. (2015). Wnt/β-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development. Development 142(19): 3383-3393.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, Y., Wang, Y., Yuka, O., Zhang, L. and Liu, C. (2016). Mouse Corneal Stroma Fibroblast Primary Cell Culture. Bio-protocol 6(19): e1960. DOI: 10.21769/BioProtoc.1960.
Category
Cell Biology > Cell isolation and culture > Cell growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link