- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluation of Angiogenesis Inhibitors Using the HUVEC Fibrin Bead Sprouting Assay
Published: Vol 6, Iss 19, Oct 5, 2016 DOI: 10.21769/BioProtoc.1947 Views: 15317
Reviewed by: Lee-Hwa TaiAnita UmeshAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cell Surface Protein-protein Binding on COS-7 Cells
Kae-Jiun Chang and Matthew N. Rasband
Jan 5, 2014 11235 Views

Automated Analysis of Cell Surface Ruffling: Ruffle Quantification Macro
Nicholas D. Condon [...] Adam A. Wall
Jan 20, 2020 5198 Views

Whole-Mount Visualization of Primary Cilia in the Developing Mouse Brain
Oscar Torres Gutierrez [...] Xuecai Ge
Dec 20, 2025 1012 Views
Abstract
Angiogenesis, the growth of new blood vessels from pre-existing vessels, is a critical process that occurs during normal development and tumor formation. Targeting tumor angiogenesis by blocking the activity of vascular endothelial growth factor (VEGF) has demonstrated some clinical benefit; nevertheless there is a great need to target additional angiogenic pathways. We have found that the human umbilical vein endothelial cell (HUVEC) fibrin bead sprouting assay (FBA) is a robust and predictive in vitro assay to evaluate the activity of angiogenesis inhibitors. Here, we describe an optimized FBA protocol for the assessment of biological inhibitors of angiogenesis and the automated quantification of key endpoints.
Background
Angiogenesis, the growth of new blood vessels from pre-existing vessels, is a physiological process that occurs during wound healing and normal development. Angiogenesis is a complex and highly regulated process involving the tight coordination of endothelial cell proliferation, differentiation, migration, matrix adhesion, and cell-to-cell signaling. Angiogenesis is also critically involved in tumor development and metastasis. Indeed, targeting tumor angiogenesis by blocking the activity of vascular endothelial growth factor (VEGF) has demonstrated clinical benefit. Since tumors do eventually develop resistance to VEGF-targeted therapy, there is a great need to target additional angiogenic pathways. We have found that the human umbilical vein endothelial cell (HUVEC) fibrin bead sprouting assay (FBA) (Nakatsu et al., 2007; Nakatsu and Hughes, 2008; Nehls and Drenckhahn, 1995) is a robust and predictive in vitro assay to evaluate the activity of angiogenesis inhibitors. This assay recapitulates key aspects of angiogenesis such as lumen formation, endothelial cell polarization and dependency on stromal cells, and is correlative with the activities of angiogenesis inhibitors as observed in in vivo tumor studies (Figures 1 and 2) (Eichten et al., 2013; Holash et al., 2012; Kuhnert et al., 2015; Noguera-Troise et al., 2006). Here we describe an optimized FBA protocol for the assessment of biological inhibitors of angiogenesis and the automated quantification of key endpoints, such as the number of endothelial cells or branch points, as well as sprout length and area (Figure 3). To illustrate the spectrum of treatment outcomes in the FBA, the effects of three different angiogenesis inhibitors [aflibercept, Dll4 blocking monoclonal antibody (Dll4 MAB) and anti-Integrin a6 antibody GOH3] on endothelial sprouting have been included in the protocol.
Materials and Reagents
- 50 ml conical tubes (Corning, Falcon®, catalog number: 352070 )
- Cytodex-3 beads (GE Healthcare, Amersham Pharmacia Biotech, catalog number: 17-0485-01 )
- Aspirator
- FACS tubes (5 ml) (Corning, Falcon®, catalog number: 352063 )
- 15 ml conical tubes (Conring, Falcon®, catalog number: 352099 )
- 1.5 ml centrifuge tube
- 24 well plate (Corning, Falcon®, catalog number: 351147 )
- 0.22 μm filter (VWR, catalog number: 28145-501 )
- Human umbilical vein endothelial cells (HUVEC) and HUVEC complete media (Lonza, catalog numbers: C2517A and CC3162 )
Note: Optimal at passage 2-4. - Normal human lung fibroblasts (NHLF) (Lonza, catalog number: CC2512 )
- Sigmacote siliconizing reagent (Sigma-Aldrich, catalog number: SL2-25ML )
- Distilled water (Thermo Fisher Scientific, GibcoTM, catalog number: 15230-162 )
- DPBS (Thermo Fisher Scientific, GibcoTM, catalog number: 14040-141 )
- Fibrinogen from bovine plasma (Sigma-Aldrich, catalog number: F8630-1G )
- Thrombin from bovine plasma (Sigma-Aldrich, catalog number: T3399-1KU )
Note: This product has been discontinued. - Aprotinin (Sigma-Aldrich, catalog number: A1153-10MG )
- Clonetics EGM-2 bullet kit (Lonza, catalog number: CC3162 )
- Trypsin/EDTA (0.025% trypsin/0.75 mM EDTA) (EMD Millipore, catalog number: SM-2004-C )
- Aflibercept (VEGF-Trap) (Regeneron Pharmaceuticals)
- Dll4 blocking monoclonal antibody (Dll4 MAB) (Regeneron Pharmaceuticals)
- Rat anti-human integrin α-6 antibody, GoH3 (BD, BD PharmingenTM, catalog number: 555734 )
- Paraformaldehyde (PFA) (16%) (Electron Microscopy Sciences, catalog number: 15710 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787-100ML )
- Phalloidin-Tetramethylrhodamine B isothiocyanate (Phalloidin-TRITC) (Sigma-Aldrich, catalog number: P1951-.1MG )
- FGM-2 bullet kit (Lonza, catalog number: CC3132 )
- Hoechst 33258, pentahydrate, bis-benzimide (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: H3569 )
- Fibrinogen solution (see Recipes)
- Thrombin stock solution (see Recipes)
- Aprotinin stock solution (see Recipes)
Equipment
- Siliconized glass bottles (Corning, PYREX®, catalog number: 1395-100 )
- Laminar flow hood
- Water bath (PolyScience, catalog number: WB05A11B )
- T25 flasks (Corning, catalog number: 430639 )
- Centrifuge
- Incubator
- P1000 pipette
- 24 well glass bottom sensoPlate (Greiner Bio One, catalog number: 662892 )
- Cell counter (Nexcelom BioScience, model: Cellometer Auto 1000 )
- Microscope (Nikon, model: Eclipse Ti-S )
- ImageXpress® MICRO XL (Molecular Devices)
Software
- MetaXpress (Molecular Devices)
Note: MetaXpress software (MX) from Molecular Devices is optimized to perform with the ImageXpressMICRO imaging systems. MX is used both to control image acquisition and to perform image analysis. Necessary features of MX utilized in FBA image acquisition and analysis are: 1) Image acquisition: Laser-based autofocus, Z-stack acquisition; 2) Image analysis: 'Tube Formation' image analysis application module, an interactive Custom Module.
Procedure
- Preparation of reagents
- Siliconization and sterilization
- Add 5 ml of Sigmacote to clean glass bottles in order to prevent the beads from sticking to the glassware.
- Rotate the vessel or glassware to ensure that the Sigmacote covers the entire surface of the glass bottle.
- Aspirate excess Sigmacote from the glassware and allow to air dry in a laminar flow hood.
- Thoroughly wash the glassware in deionized tissue culture grade water. A minimum of two washings is suggested.
- Sterilize glassware by autoclaving.
- Add 5 ml of Sigmacote to clean glass bottles in order to prevent the beads from sticking to the glassware.
- Cytodex bead preparation
- In a 50 ml Falcon tube, hydrate 0.5 g dry beads in 50 ml PBS (pH = 7.4). Place on a rocker for at least 3 h at RT.
- Let beads settle (~15 min). Aspirate the supernatant using a pipette and wash 3 x 5 min in 50 ml fresh PBS using a rocker at RT. Do not vortex.
- Aspirate PBS using a pipette and replace with fresh PBS (50 ml) such that the bead concentration is 10 mg/ml or 30,000 beads/ml.
- Transfer the bead suspension in a siliconized glass bottle.
- Sterilize the beads by autoclaving for 15 min at 115 °C.
- Store at 4 °C.
- In a 50 ml Falcon tube, hydrate 0.5 g dry beads in 50 ml PBS (pH = 7.4). Place on a rocker for at least 3 h at RT.
- Fibrinogen solution preparation (see Recipes)
- Thrombin stock solution preparation (see Recipes)
- Aprotinin stock solution preparation (see Recipes)
- Siliconization and sterilization
- Preparation of cells
Change the growth media for HUVECs and fibroblasts to EGM-2 media 1 day before use. For HUVECs, switch medium to EGM-2 the day before beading. For fibroblasts, switch the FGM-2 medium to EGM-2 the day before embedding in fibrin. Beading requires ~400 HUVECs per bead. Use 20,000 fibroblasts per well of a 24-well plate. - Coating the beads with HUVEC (Day-1)
- Warm EGM-2 media in a water bath set to 37 °C.
- Trypsinize HUVECs which should be 80% confluent. Wash cells with PBS and then add 3 ml of trypsin. Allow trypsin to remain on cells for approximately 2 min or until cells begin to detach from the flask. Add EGM-2 media to neutralize the trypsin and bring the total volume to 10 ml. Spin cells in a centrifuge, remove supernatant with an aspirator, and adjust cell concentration to 2 x 106 cells/ml.
- Under sterile conditions, place 170 μl bead solution (stock concentration: 30,000 beads/ml) in a FACS tube. This should be ~5,000 beads. Allow beads to settle (do not centrifuge), aspirate the supernatant, and wash the beads in 2 ml of warm EGM-2 medium. Remove media using a pipette and add fresh warm media for a total volume of 2 ml.
- Add 2 x 106 HUVEC (1 ml of 2 x 106 cells/ml) to 5,000 beads (in 2 ml) in the FACS tube (total volume 3 ml). Place tube vertically in the incubator. This will be enough for ~20 wells.
- Incubate for 4 h at 37 °C, inverting and mixing the tube every 20 min. Beads should look like mini golf balls after beading (Figure 1).
- After 4 h, transfer the coated beads to a T25 tissue culture flask and leave overnight in a total volume of 5 ml of EGM-2 at 37 °C and 5% CO2.
- Warm EGM-2 media in a water bath set to 37 °C.
- Embedding coated beads in fibrin gel (Day 0)
- Add 0.15 Units/ml of aprotinin to 15 ml of fibrinogen solution.
- Check HUVEC coated beads. Transfer 5 ml of coated beads to a 15 ml conical tube and let the beads settle. Use additional 5 ml of media to wash off beads that are stuck to flask.
- Let beads settle to the bottom of the tube. Aspirate off media. Re-suspend beads in 1 ml of EGM-2 and transfer to a 1.5 ml centrifuge tube.
- Wash the beads 2 x with 1 ml of EGM-2, mixing by pipetting up and down very slowly/carefully (beads are fragile) with a P1000 pipette.
- Add 6.25 μl of thrombin stock solution (50 U/ml) to the center of each well of a 24-well plate. This will result in 0.625 U/ml once 0.5 ml of fibrinogen/bead suspension is added in the next step.
- Get fibrinogen/bead mixture into solution by pipetting up and down gently. Add 0.5 ml of fibrinogen/bead suspension to each well.
- Mix the thrombin and the fibrinogen/beads by pipetting up and down gently ~five times. Change the pipette tip for each well in order to prevent clotting in the pipette tip between wells. Avoid creating bubbles in the fibrin gel (may hinder visibility in gel). Do not move the plate in order to avoid tearing of the fibrin gel.
Note: Usually, when the fibrin gel is formed, tiny bubbles will be present in the gel. They will disappear in 3 to 4 days. - Allow the fibrinogen/bead solution to solidify for 5 min at RT in hood. It is important that the plate is not disturbed during the first 5 min of clotting because sheared fibrin reduces sprouting. After 5 min, check under microscope to see if beads are evenly distributed (not clumped together). Ideally there should be 200-250 beads per well.
Note: Increasing the number of beads per well results in earlier anastomosis. - Then incubate at 37 °C and 5% CO2 for 10-15 min.
- While waiting, trypsinize fibroblasts and re-suspend in EGM adjusted to 1 x 106 cell/ml (or 1,000 cells/μl)
Note: Fibroblasts will proliferate, migrate and form a monolayer on the fibrin gel. - Prepare the appropriate amount of EGM-2 media containing the angiogenesis inhibitor of choice (aflibercept at 50 μg/ml, Dll4 MAB at 50 μg/ml, Integrin α-6 antibody GoH3 at 10 μg/ml).
- After incubation, add 1 ml of EGM-2 prepared to each well slowly (drop by drop). If media is added too fast, it could tear the gel.
- Seed 20 μl of NHLF cell solution (20,000 cells) on top of the clot into each well. Add directly into the center of well and into the media.
- Replace with fresh EGM-2 medium every other day (day 2, 5, 7, etc.) until desired growth (see notes below) is achieved and capture images accordingly.
Notes:- Budding/sprouting should be apparent between day 2 and 4.
- Lumen formation begins around day 4 to 5 and sprouts continue to elongate.
- Newly formed tubes begin to branch around day 4 to 6.
- By day 6 to 7, the microvessel-like structures begin to anastomose (connection of two structures) with adjoining tubes.
- Budding/sprouting should be apparent between day 2 and 4.
- Add 0.15 Units/ml of aprotinin to 15 ml of fibrinogen solution.
- Gel processing and sprout staining for DNA and actin
- To stop the assay, 500 μl of 2% paraformaldehyde (PFA) is added to the gels for 1 h at 37 °C, followed by overnight incubation at room temperature (RT). Wash twice with PBS.
- To stain the endothelial sprouts, gels are permeabilized with 500 μl of 0.5% Triton X-100 for 20 min, washed twice with PBS.
- Incubate with the mixture of 500 μl of Phalloidin-TRITC (1:1,000) and Hoechst 33258 (1:2,000) in PBS for 1 h at RT.
- Wash twice with PBS.
- To stop the assay, 500 μl of 2% paraformaldehyde (PFA) is added to the gels for 1 h at 37 °C, followed by overnight incubation at room temperature (RT). Wash twice with PBS.
- Image acquisition on ImageXpressMICRO XL
- Fibrin beads assays are performed in 24 well optical plates (Sensoplate).
- Images of whole wells are acquired with 10x objective using automated microscope ImageXpressMICRO XL and MetaXpress (MX) software, as follows: for each site, proprietary Z-stack journal is used to collect sixteen focus planes separated by 10 μm. Proprietary Z-stack journal is a configured journal (macro) written in MX software. The configured journal consists of a 'command' guiding the stage move in Z-plane, followed by a command guiding collection of 16 images separated by the distance of 10 μm, followed by a command to create max. intensity projection of these 16 images.
- Planes are collapsed into one final image using MX Maximum Intensity Projection algorithm.
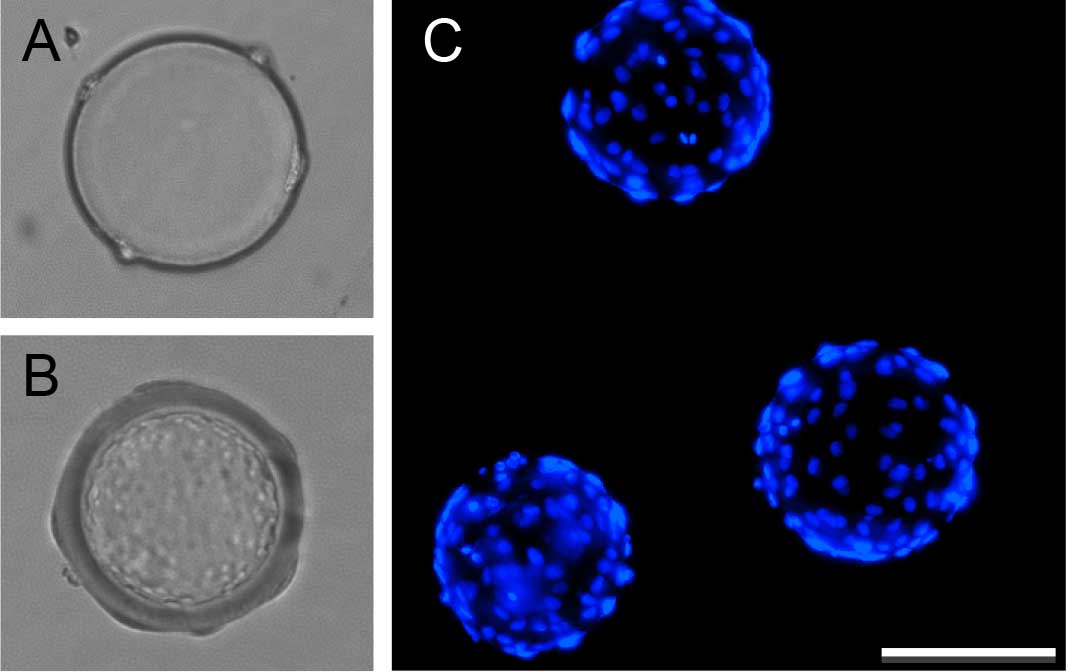
Figure 1. Coating Cytodex beads with human umbilical vein endothelial cells. Bright field images of Cytodex beads before (A) and after coating with endothelial cells (B). Hoechst 33258 staining identifies endothelial nuclei on coated beads (C). Scale bar represents 100 μm.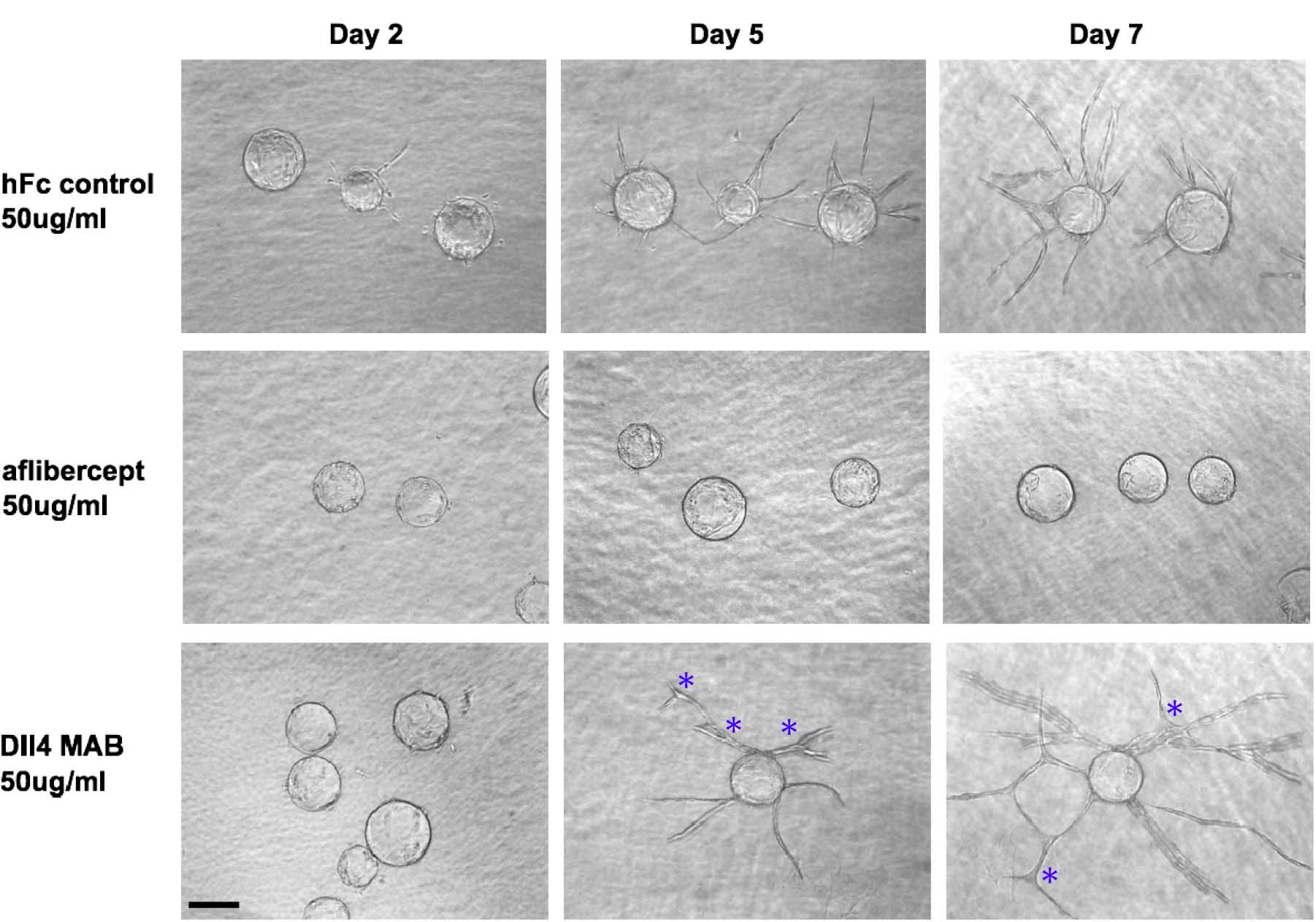
Figure 2. Effects of the angiogenesis inhibitors aflibercept and Dll4 MAB on HUVEC sprouting. Aflibercept treatment quantitatively suppressed HUVEC sprouting, while blockade of Dll4 with Dll4 MAB increased HUVEC sprout length and branch point number. Scale bar represents 100 μm. Asterisks mark branch points.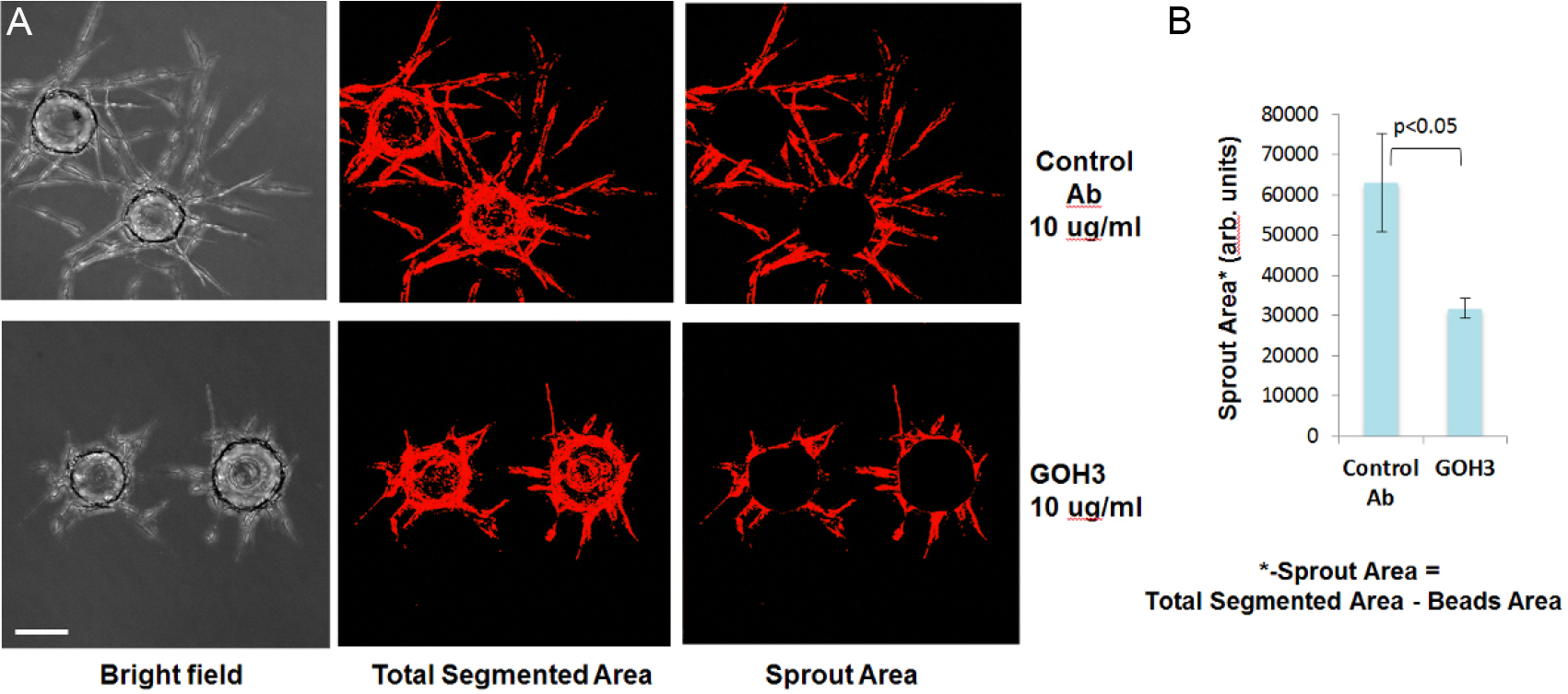
Figure 3. Integrin α-6 antibody GOH3 significantly inhibits HUVEC sprouting. A. Representative images of stained sprouts in transmitted light channel (left panel) or TRITC channel (middle and right panels) are shown. Fluorescent images of fixed gels were acquired on ImageXpressMICRO automated microscope. Scale bar represents 100 μm. B. Sprout Area image (right panel) was derived by subtracting a circular shape corresponding to Bead Area from Total Segmented Area Image (Figure 3A, middle panel) using 'Subtract' function of MetXpress software.
- Fibrin beads assays are performed in 24 well optical plates (Sensoplate).
Data analysis
- Image analysis using MetaXpressTM software (MX): To calculate the number of endothelial cells per bead, maximum intensity projection images acquired in transmitted light and DAPI channels were analyzed using configured MX Custom Module in combination with configured MX Multi-Wavelength Cell Scoring Module (Kuhnert et al., 2015; Sweet et al., 2012).
- To calculate the total length of sprouts, or the number of branch points, Maximum Intensity Projection images acquired in transmitted light and DAPI channels were analyzed using configured MX Custom Module in combination with configured MX Tube Formation Module. Statistical significance for quantitative data is determined by Student’s t-test in pairwise comparisons and one-way ANOVA for multiple comparisons (Kuhnert et al., 2015; Sweet et al., 2012).
Recipes
- Fibrinogen solution
Dissolve 2 mg/ml fibrinogen in DPBS in a 37 °C water bath.
Mix by inverting the tube.
Do not vortex.
Pass through a 0.22 μm filter to sterilize. - Thrombin stock solution
Reconstitute in sterile water at 50 U/ml and sterile filter through a 0.22 μm filter.
Make aliquots of 0.5 ml each and store at -20 °C. - Aprotinin stock solution
Reconstitute lyophilized aprotinin at 4 U/ml in DI water and sterile filter through a 0.22 μm filter.
Make aliquots of 0.5 ml each.
Store at -20 °C.
Acknowledgments
This work was funded by Regeneron Pharmaceuticals, Inc.
References
- Eichten, A., Adler, A. P., Cooper, B., Griffith, J., Wei, Y., Yancopoulos, G. D., Lin, H. C. and Thurston, G. (2013). Rapid decrease in tumor perfusion following VEGF blockade predicts long-term tumor growth inhibition in preclinical tumor models. Angiogenesis 16(2): 429-441.
- Holash, J., Davis, S., Papadopoulos, N., Croll, S. D., Ho, L., Russell, M., Boland, P., Leidich, R., Hylton, D., Burova, E., Ioffe, E., Huang, T., Radziejewski, C., Bailey, K., Fandl, J. P., Daly, T., Wiegand, S. J., Yancopoulos, G. D. and Rudge, J. S. (2002). VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 99(17): 11393-11398.
- Kuhnert, F., Chen, G., Coetzee, S., Thambi, N., Hickey, C., Shan, J., Kovalenko, P., Noguera-Troise, I., Smith, E., Fairhurst, J., Andreev, J., Kirshner, J. R., Papadopoulos, N. and Thurston, G. (2015). Dll4 blockade in stromal cells mediates antitumor effects in preclinical models of ovarian cancer. Cancer Res 75(19): 4086-4096.
- Nakatsu, M. N., Davis, J. and Hughes, C. C. (2007). Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp(3): 186.
- Nakatsu, M. N. and Hughes, C. C. (2008). An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol 443: 65-82.
- Nehls, V. and Drenckhahn, D. (1995). A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res 50(3): 311-322.
- Noguera-Troise, I., Daly, C., Papadopoulos, N. J., Coetzee, S., Boland, P., Gale, N. W., Lin, H. C., Yancopoulos, G. D. and Thurston, G. (2006). Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444(7122):1032-1037.
- Sweet, D. T., Chen, Z., Wiley, D. M., Bautch, V. L. and Tzima, E. (2012). The adaptor protein Shc integrates growth factor and ECM signaling during postnatal angiogenesis. Blood 119(8): 1946-1955.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Winters, L., Thambi, N., Andreev, J. and Kuhnert, F. (2016). Evaluation of Angiogenesis Inhibitors Using the HUVEC Fibrin Bead Sprouting Assay. Bio-protocol 6(19): e1947. DOI: 10.21769/BioProtoc.1947.
Category
Cancer Biology > Angiogenesis > Cancer therapy
Cell Biology > Cell structure > Cell surface
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link