- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
ASC-particle-induced Peritonitis
Published: Vol 6, Iss 19, Oct 5, 2016 DOI: 10.21769/BioProtoc.1944 Views: 7175
Reviewed by: Ivan ZanoniAchille BroggiMarco Di Gioia

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2530 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2552 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3991 Views
Abstract
In response to pathogen infection and tissue damage, inflammasome sensors such as NLRP3 and AIM2 are activated, which triggers PYRIN domain (PYD)-mediated ASC nucleation, followed by self-perpetuating ASC polymerization, which ultimately culminates in caspase-1 activation, interleukin (IL)-1β and IL-18 processing and release and pyroptosis (Ratsimandresy et al., 2013; Cai et al., 2014). Inflammasomes release not only cytokines, but also the polymeric ASC danger particles (pASC) by pyroptosis, which perpetuate and propagate inflammasome responses to bystander cells to engage cell intrinsic ASC and caspase-1 (Baroja-Mazo et al., 2014; Franklin et al., 2014). In this protocol we describe intraperitoneal injection of polymeric ASC particles as a danger signal and measure neutrophil infiltration and levels of the pro-inflammatory cytokine IL-1β by ELISA in the peritoneal lavage (de Almeida et al., 2015).
Keywords: InflammasomeMaterials and Reagents
- 5 ml syringes (BD, catalog number: 309646 )
- 25 G x1 ½ needles (BD, catalog number: 305127 )
- Conical tubes (15 ml) (Corning, Falcon®, catalog number: 352095 )
- C57BL/6 mice, typically of 8-12 weeks old (male or female)
- 1 x 105 pASC-GFP particles generated from stable or transiently expressing HEK293 cells and sorted by flow cytometry as described (Fernandes-Alnemri et al., 2007; Fernandes-Alnemri and Alnemri, 2008)
- 1x Dulbecco's phosphate-buffered saline (DPBS) (Corning, catalog number: 21-030-CV )
- HEPES
- KOH
- Magnesium chloride (MgCl2)
- EGTA
- CHAPS
- cOmplete protease inhibitor cocktail (Roche Diagnostics, catalog number: 11697498001)
- IL-1β ELISA kit (BD, catalog number: 559603 )
- Lysis buffer (see Recipes)
Equipment
- Centrifuge
- Fluorescence microscope
- Flow cytometry
- Surgical instruments such as tweezers and scissors
- Biosafety cabinet
Procedure
- Isolate pASC-GFP particles from 107 HEK293 cells stably or transiently transfected with ASC-GFP as described previously (Fernandes-Alnemri et al., 2007; Fernandes-Alnemri and Alnemri, 2008; Martín-Sánchez et al., 2015) or by flow cytometry.
- Remove media from cells, add DPBS and gently scrape cells. Spin down cells at 1,500 x g for 5 min. Discard supernatant.
- Prepare total cell lysates by hypotonic lysis of cell pellets in 20 mM HEPES-KOH, pH 7.5, 5 mM MgCl2, 0.5 mM EGTA, 0.1% CHAPS, supplemented with protease inhibitors. Use a syringe with 25 G needle to lyse the cells by syringing several times. Centrifuge cell lysates full speed to obtain cell lysates supernatant.
- Induce aggregation of ASC-GFP by incubation of cell lysates supernatant at 37 °C for 30 min as previously described (Fernandes-Alnemri et al., 2007; Fernandes-Alnemri and Alnemri, 2008). The typical number of ASC-GFP particles recovered is between 2 x 105 and 4 x 105 per million cells.
- Add a small amount of supernatant to a microscope slide to confirm ASC polymerization into 1-3 μm aggregates by fluorescence microscopy using a standard GFP excitation/emission filter set (excitation: 484 nm; emission: 507 nm) (Figure 1).
- Sort ASC-GFP particles (1-2 μm) by flow cytometry by gating for small particles based on forward scatter versus side scatter. Next start by gating for FITC positive particles. Later confirm ASC-GFP particles by fluorescent microscopy (Figure 1). Particles can be stored at 4 °C for one year.
- Intraperitoneally inject mice with 1 x 105 pASC-GFP particles or the same volume DPBS for control mice (injection volume approximately 200 μl).
- 4 h later sacrifice mice, cut the skin and expose the peritoneal wall (Video 1).Video 1. Peritoneal lavage. The video shows how to wash peritoneal cavity after injection of pASC-GFP particles to measure IL-1β by ELISA.
- Inject 4 ml of DPBS into the peritoneal cavity.
- Shake the mouse to wash the peritoneal cavity.
- Use the syringe to recover DPBS injected by gradually pulling out the plunge.
- Remove the needle and add content to a conical tube.
- Spin down the cells at 1,200 x g for 5 min.
- Transfer supernatant to fresh conical tubes and measure IL-1β by ELISA. Typical IL-1β concentrations are up to 2 ng/ml and a representative result is shown in our previous publication (de Almeida et al., 2015).
Representative data
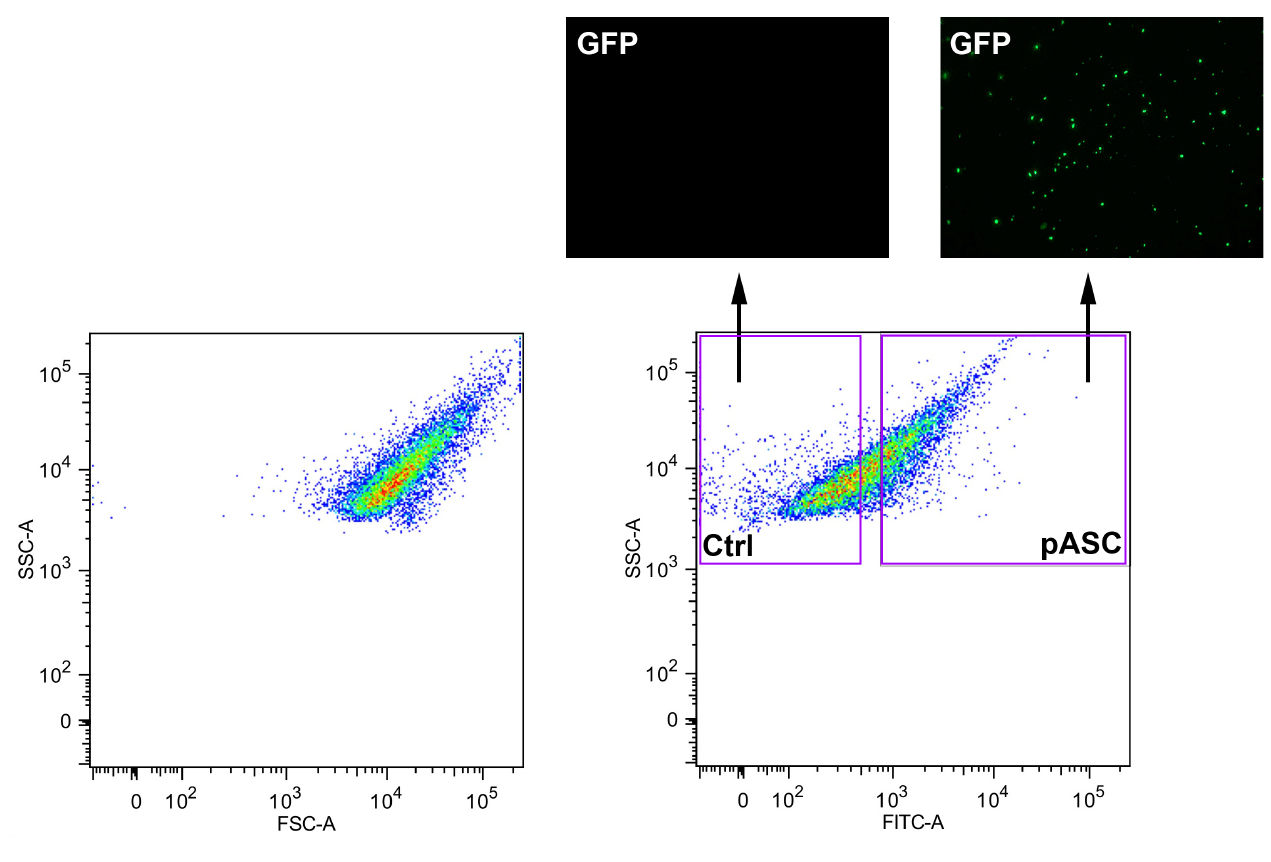
Figure 1. Total cell lysates from stable ASC-GFP-expressing HEK293 cells were GFP-sorted by flow cytometry after inducing ASC polymerization and control (Ctrl) and ASC-GFP (pASC)-containing fractions analyzed by fluorescence microscopy. HEK293 cell lysates were used as a negative control.
Notes
- ASC-GFP particles should be protected from light until use.
Recipes
- Lysis buffer
20 mM HEPES-KOH, pH 7.5
5 mM MgCl2
0.5 mM EGTA
0.1% CHAPS
Protease inhibitors
Acknowledgments
This protocol was adapted from a previously published study (de Almeida et al., 2015). This work was supported by grants the National Institutes of Health (AI099009, AI120625, AI120618 and AR064349 to C.S., AR066739 to A.D., AI120625 and AI120618 to C.S. and A.D., T32AR007611 to L.d.A. and the American Heart Association 13GRNT17110117 to C.S.).
References
- Baroja-Mazo, A., Martin-Sanchez, F., Gomez, A. I., Martinez, C. M., Amores-Iniesta, J., Compan, V., Barbera-Cremades, M., Yague, J., Ruiz-Ortiz, E., Anton, J., Bujan, S., Couillin, I., Brough, D., Arostegui, J. I. and Pelegrin, P. (2014). The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15(8): 738-748.
- Bondar, V. S. and Puzyr, A. P. (2000). Use of nanodiamond particles for rapid isolation of recombinant apoobelin from Escherichia coli. Dokl Biochem 373(1-6): 129-131.
- Cai, X., Chen, J., Xu, H., Liu, S., Jiang, Q. X., Halfmann, R. and Chen, Z. J. (2014). Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156(6): 1207-1222.
- de Almeida, L., Khare, S., Misharin, A. V., Patel, R., Ratsimandresy, R. A., Wallin, M. C., Perlman, H., Greaves, D. R., Hoffman, H. M., Dorfleutner, A. and Stehlik, C. (2015). The PYRIN domain-only protein POP1 inhibits inflammasome assembly and ameliorates inflammatory disease. Immunity 43(2): 264-276.
- Fernandes-Alnemri, T., Alnemri, E. S. (2008). Chapter thirteen assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol 442: 251-270.
- Fernandes-Alnemri, T., Wu, J., Yu, J. W., Datta, P., Miller, B., Jankowski, W., Rosenberg, S., Zhang, J. and Alnemri, E. S. (2007). The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14(9): 1590-1604.
- Franklin, B. S., Bossaller, L., De Nardo, D., Ratter, J. M., Stutz, A., Engels, G., Brenker, C., Nordhoff, M., Mirandola, S. R., Al-Amoudi, A., Mangan, M. S., Zimmer, S., Monks, B. G., Fricke, M., Schmidt, R. E., Espevik, T., Jones, B., Jarnicki, A. G., Hansbro, P. M., Busto, P., Marshak-Rothstein, A., Hornemann, S., Aguzzi, A., Kastenmuller, W. and Latz, E. (2014). The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat Immunol 15(8): 727-737.
- Martín-Sánchez, F., Gómez, A. I. and Pelegrín, P. (2015). Isolation of particles of recombinant ASC and NLRP3. Bio-protocol 5(10): e1480.
- Ratsimandresy, R. A., Dorfleutner, A. and Stehlik, C. (2013). An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Front Immunol 4: 440.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
de Almeida, L., Dorfleutner, A. and Stehlik, C. (2016). ASC-particle-induced Peritonitis. Bio-protocol 6(19): e1944. DOI: 10.21769/BioProtoc.1944.
Category
Immunology > Animal model > Mouse
Cell Biology > Cell signaling > Stress response
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link