- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Skin TRITC Painting to Track Dendritic Cells Migrating to the Lymph Nodes
Published: Vol 6, Iss 18, Sep 20, 2016 DOI: 10.21769/BioProtoc.1939 Views: 9642
Reviewed by: Ivan ZanoniAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2530 Views

In Vitro Bone Marrow–Derived Dendritic Cells (BMDC) Generation for Antigen Presentation Assay
Sudhakar Singh [...] Sharvan Sehrawat
Apr 20, 2025 4352 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3991 Views
Abstract
Our protocol describes a simple method that allows tracking of dendritic cells (DC) migration from the flank skin to draining lymph nodes (LN) using a red fluorescent dye tetramethylrhodamine-5-isothiocyanate (TRITC). TRITC is a photostable dye that readily labels cells including DC in the skin and can survive repeated exposure of two-photon laser excitation for prolonged imaging duration. This method can be combined with various fluorescent antibody labels or transgenic mouse strains (such as CD11c-EYFP) to visualize distinct DC populations simultaneously.
Keywords: Dendritic cellsMaterials and Reagents
- 10-20 μl pipette tips
- Paraffin film (Cole-Parmer, catalog number: PM996 )
- Eppendorf tubes (Eppendorf, catalog number: 022364111 )
- Felt-tip marker (Sharpie, fine point permanent marker)
- Mouse (C57BL/6)
- Tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC) (Thermo Fisher Scientific, catalog number: T490 )
- Acetone (Chem Supply, catalog number: AL008-2.5L-P )
- DMSO (Dimethyl sulfoxide) (Sigma-Aldrich, catalog number: D2650 )
- Veet (sensitive skin hair removal cream) (Reckitt Benckiser)
- Eye gel (e.g., Poly Visc, Alcon Laboratories)
- Ilium Ketamil (Ketamine) (Troy Laboratories)
- Ilium Xylazil-20 (Xylazine) (Troy Laboratories)
- Ketamine/Xylazine solution (see Recipes)
Equipment
- 10-20 μl pipette
- Spatula
- Fine balance for weighing 0.1 μg
- Wahl super trimmer (Wahl, catalog number: 5996415011081 )
- Heat pad (Stoelting, model: 50300 )
- Clippers
- Tissue paper/gauze
- Ruler
Procedure
- Preparing TRITC solution
Note: TRITC solution is prepared fresh prior to use.- Weigh a small amount (e.g., 0.1 μg) of TRITC crystals onto a square Parafilm. The amount of TRITC prepared is dependent on the volume required for use (see step B7).
- Dissolve in 1 μl DMSO per 0.1 μg of TRITC on Parafilm.
- Prepare acetone in an Eppendorf tube and transfer the dissolved TRITC/DMSO solution into the acetone to dilute to 0.5-1% (v/v) (e.g., 1-2 μl TRITC/DMSO in 200 μl acetone).
- Mix tube thoroughly with a pipette.
- Weigh a small amount (e.g., 0.1 μg) of TRITC crystals onto a square Parafilm. The amount of TRITC prepared is dependent on the volume required for use (see step B7).
- TRITC painting
- Anaesthetize a mouse (e.g., C57BL/6) with Ketamine/Xylazine (10 μl per g bodyweight) via intraperitoneal injection.
Notes:- This procedure should be applicable to all mouse strains used for research.
- All procedures involving animals must be performed according to approved institutional, regional or national protocols (as applicable) by appropriately trained and certified personnel.
- This procedure should be applicable to all mouse strains used for research.
- Lay sedated mouse onto heat pad and apply one drop of eye gel to each eye.
- Shave the flank skin of the mouse using clippers.
Note: We have tested this method on the flank/back skin of mice, however it should be applicable to all other skin sites after appropriate depilation. - Apply Veet to shaved skin for 2 min to thoroughly depilate hair. Wipe off the cream with tissue paper or gauze.
- Clean the exposed skin with tissue paper or gauze soaked with water, and dry the skin with dry tissue paper.
- With a felt-tip marker and ruler, mark the area on skin to be painted (Figure 1, left). To prevent ink from being absorbed where TRITC is applied, mark the skin ~2-3 mm wider than the desired size.
- With a P20 pipette, mix the TRITC solution thoroughly and slowly apply onto the intended area, drop-by-drop (Figure 1, right. We use 10 μl dye/acetone mix for a ~1 cm2 diameter painting site. The volume to be used should be scaled accordingly). The dye should be absorbed into the skin within a few seconds.
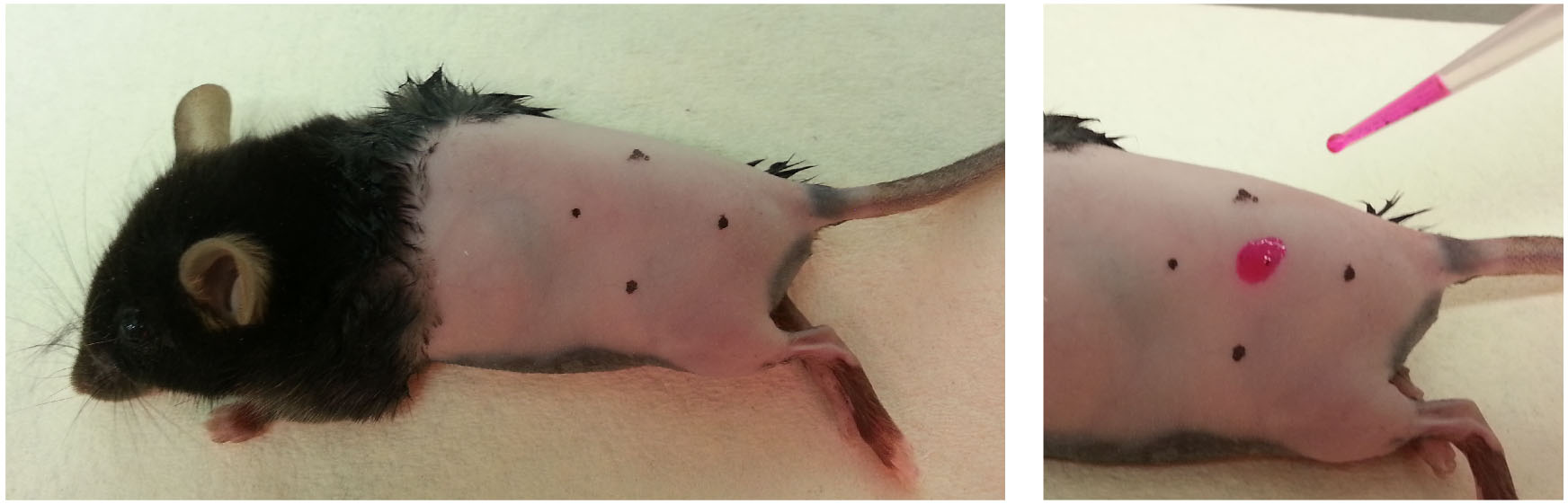
Figure 1. Preparing mouse for dye painting. Shave and clean the flank skin of mouse, mark the intended area for painting (left) and apply the dye/acetone mix slowly with a pipette onto the skin (right). - Lay mouse by its side to allow the painted site to dry (approximately 10-15 min). Leave the mouse on the heat pad until awake (approximately 1 h until recovered from anesthesia).
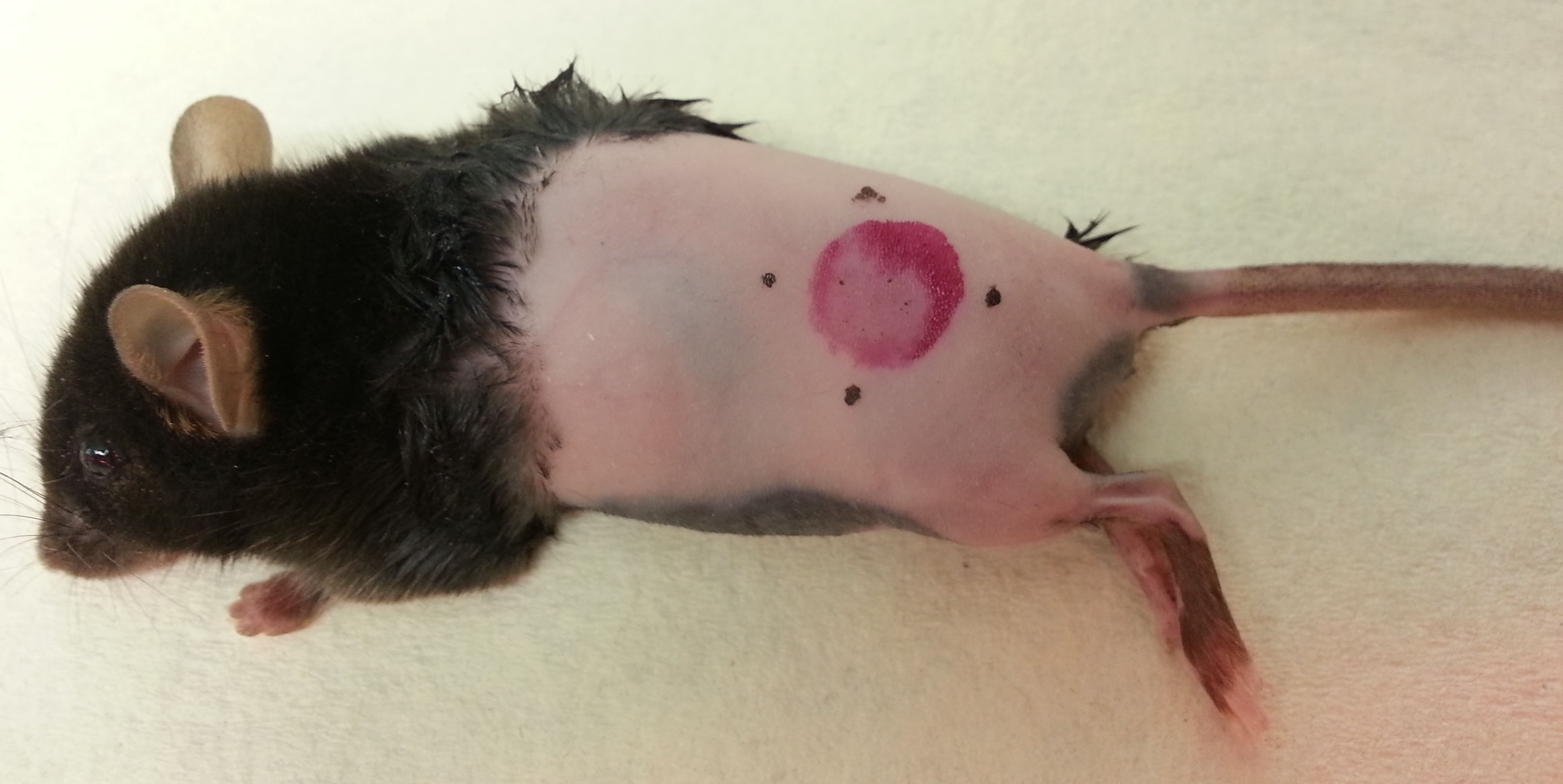
Figure 2. TRITC-painted mouse. Dye/acetone mix should be absorbed relatively quickly into the skin. Leave mouse by its side, on heat pad, to allow the painted site to dry. - Downstream applications include detection of migratory cells in draining LN using flow cytometry, immunofluorescence microscopy or two-photon intravital microscopy (Figure 3, see Notes 1 and 2 below).
- Anaesthetize a mouse (e.g., C57BL/6) with Ketamine/Xylazine (10 μl per g bodyweight) via intraperitoneal injection.
Representative data
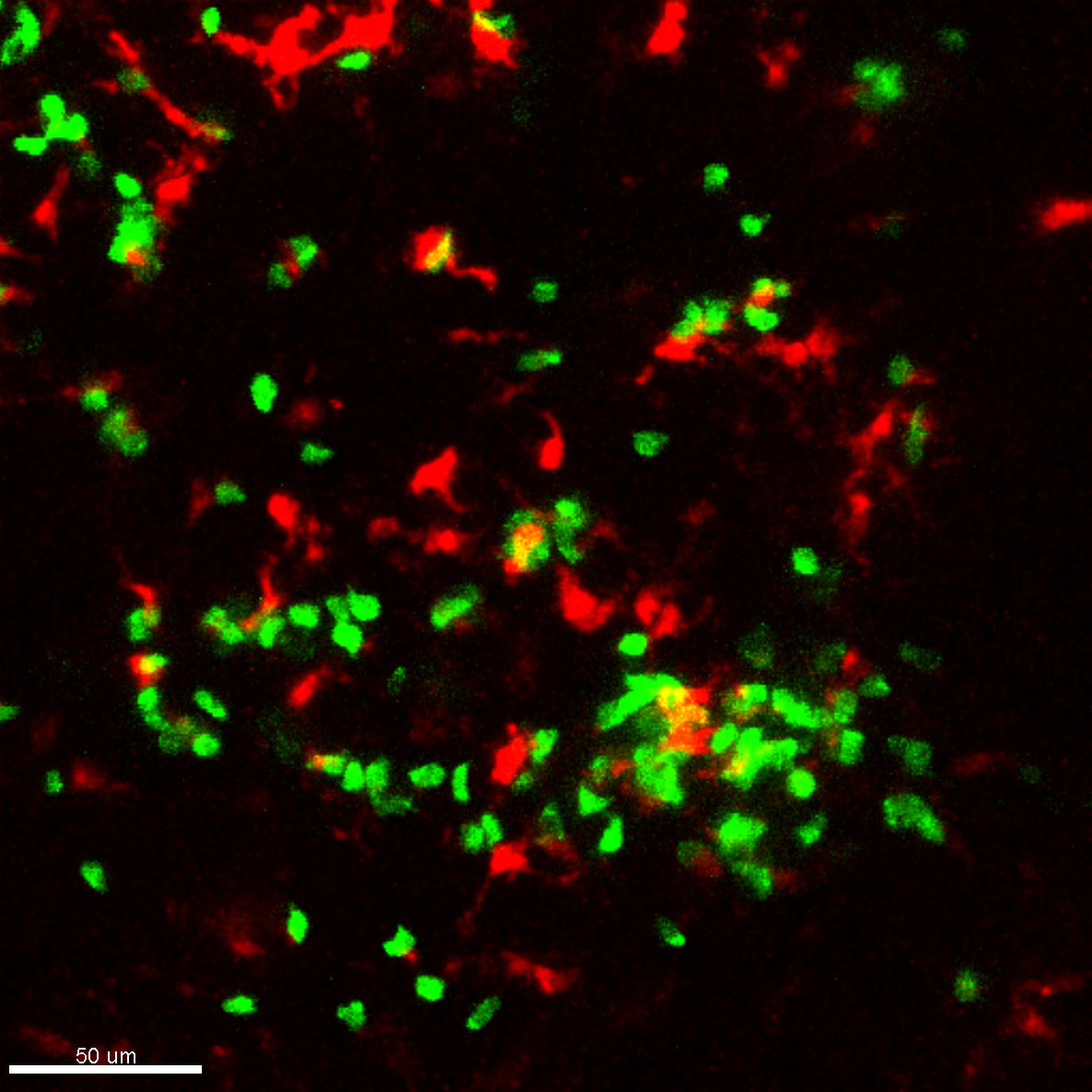
Figure 3. Example of TRITC-labelled DC imaging using two-photon intravital microscopy. Shown is a snapshot from a time lapse movie depicting TRITC-labelled migratory DC (red) and their interaction with CellTrace Violet-labelled CD4 T cells (green) in the T cell zone of mouse inguinal LN. Scale bar, 50 μm.
Notes
- The dye is photostable and can sustain repeated exposure to laser excitation.
- For two-photon imaging, TRITC is excitable from a range of 800-880 nm with a Ti:Sa laser.
- We observed only very low-level dye drainage to the draining LN, most of which was retained in the subcapsular sinuses or captured by the macrophages that line the periphery of the LN. Cells labeled by the dye, on the other hand, can be readily distinguished by their high fluorescence intensity. TRITC-painted migrants can be identified via flow cytometry or immunofluorescence confocal microscopy. In our experiments with cutaneous HSV infection, the majority of TRITChi cells in the draining LN appeared to be CD11c+ MHC-IIhi, suggesting that they are migratory DCs.
- We observed migration of cells in the draining LN a few hours following painting. This may reflect homeostatic circulation of the cells, or alternatively activation of cells triggered by the procedures performed (shaving, depilation, dye painting). Regardless of what triggered their migration, the presence of these cells did not affect our ability to detect migratory DCs arriving from skin presenting antigens after virus infection.
Recipes
- Ketamine/xylazine solution
For 10 ml solution:
1 ml Ilium Ketamil (Ketamine)
0.75 ml Ilium Xylazil-20 (Xylazine)
8.25 ml PBS
Store at 4 °C
Note: Mice are anesthetized with 10 μl per g bodyweight.
Acknowledgments
This protocol was adapted from our publication (Hor et al., 2015). This work was supported by the National Health and Medical Research Council, and by the Australian Research Council.
References
- Hor, J. L., Whitney, P. G., Zaid, A., Brooks, A. G., Heath, W. R. and Mueller, S. N. (2015). Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T Cell activation to localized viral infection. Immunity 43(3): 554-565.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hor, J. L. and Mueller, S. N. (2016). Skin TRITC Painting to Track Dendritic Cells Migrating to the Lymph Nodes. Bio-protocol 6(18): e1939. DOI: 10.21769/BioProtoc.1939.
Category
Immunology > Animal model > Mouse
Immunology > Immune cell function > Dendritic cell
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











