- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Object-context Recognition Memory Test for Mice
Published: Vol 6, Iss 17, Sep 5, 2016 DOI: 10.21769/BioProtoc.1925 Views: 10692
Reviewed by: Soyun KimEdgar Soria-GomezXi Feng

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1303 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1818 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1474 Views
Abstract
The object in-context (OIC) task is a variant of the widely used object recognition (OR) task (Dix and Aggleton, 1999). The OIC task makes use of the fact that rodents have a natural tendency to explore novel environments and objects. The hippocampus appears to play a major role in the OIC task (much more so than in the original OR task), where animals should be able to distinguish between two familiar objects of which one is in a different context from the training trial (Ennaceur and Aggleton,1997; Bermudez-Rattoni et al., 2005; Albasser et al., 2009; Roozendaal et al., 2010; Banks et al., 2014; Bermudez-Rattoni, 2014). Recognition memory encompasses a number of additional components, such as an item's associations with its context, place, etc. (Bussey et al., 1999, 2000). Here, we describe a version of the OIC task in mice, based on earlier reports (Dix and Aggleton, 1999; Eacott and Norman, 2004; Balderas et al., 2008; Barsegyan et al., 2014; Kanatsou et al., 2015a; Kanatsou et al., 2015b).
Keywords: LearningMaterials and Reagents
The protocol was approved by the committee on Animal Health and Care from the University of Amsterdam, the Netherlands (permit number: DED 291).
- Standard sawdust bedding (just enough to cover the bottom of the box)
Note: The amount of bedding should be enough to cover the bottom of the context to reduce anxiety behavior. However, this may interfere with other ethological behaviours (e.g., digging), which in turn makes the mouse not to focus on the object but instead with the bedding. You can also try without bedding, but you need to test this before yourself. - Mouse, C57BL/6J strain, male and female, 2-4 months old, group housed (2-4 mice per group)
Note: Mice were kept in a temperature and humidity controlled facility (21.5-22 °C, with humidity between 40 and 60%) on a 12 h light/dark cycle (lights on at 8:00 a.m.) with food and water available ad libitum. - 70% ethanol (v/v)
Note: Clean the objects using 70% ethanol. Air dry for 1 min.
Equipment
- Apparatus
For context we used four blue-colored plastic boxes of identical size (W x L x H = 33 x 54 x 37 cm) with or without visual cues on the walls. As visual cues we used tape in white color drawing cues in the walls (Figure 1).
Note: Consider a large open field apparatus for this test, because the displacement of an object is more noticeable compared to a small size apparatus, while at the same time you provide the mouse with the ability to explore the extra cues in the box.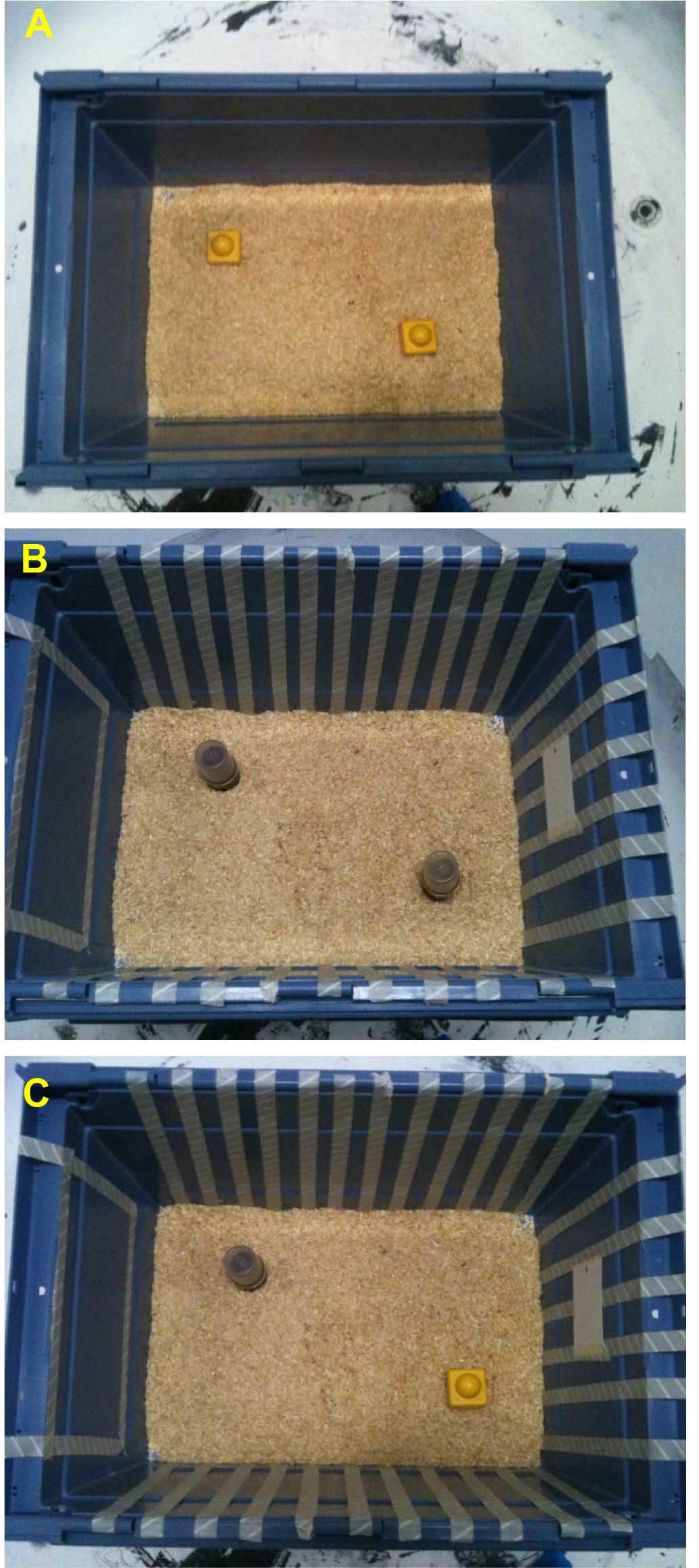
Figure 1. Images for Objects and contexts. A. Day 2: training in context A (Lego objects) for 10 min. B. Day 2: training in context B (bottle objects) - > 10 min. C. Day 3: testing in context B (replaced bottle with Lego from context A) - > 10 min. - Objects
We used blocks of Lego and/or small bottles (Figure 1).
Note: The objects should be approximately of equal size to the mouse or slightly bigger. The two set of objects should be different enough to be distinguished by the mice. The objects are recommended to be made of hard plastic or glass, so they are resistant to biting. Both two set of objects should be of approximately equal size.
Procedure
The OIC protocol is performed during the light phase and consists of three phases: 1. The phase where the animals are habituated to the exploration box. 2. The training phase where they encounter the objects for the first time (two identical objects in a specific context and two other identical object in another context). 3. The testing phase where one of the identical objects in the context is replaced by the other object. The animals should be able to distinguish between the familiar object in the same context and the familiar object in the non-matching context. This would result in more exploratory behaviour towards the object that is replaced in the non-matching environment since this is the “new” scenario.
Mice are tested on three subsequent days:
- Day 1. Habituation
The mouse is placed for 10 min in the context (with its face towards the wall) with no wall cues and without objects (Figure 2A). - Day 2. Training
On day 2, the mouse is placed for 10 min in a box (context A) that has no cues on the walls but contained two identical objects, i.e., two blocks of Lego, placed in opposite corners (Figure 2B.1). Thereafter (approximately 1 min retention in the home cage), the mouse is placed for 10 min into another box (context B) with cues on the walls in the form of stripes and which contains two (new) identical objects, i.e., 2 small bottles, placed in opposite corners (Figure 2B.2). After training in context B, the mouse returns to its own home cage.
Note: The duration (in sec) that mice spent to sniff the object (Figure 3). - Day 3. Testing
On day 3, the mouse is placed for 10 min in context B containing one object which was present in context B on day 2 (i.e., familiar object to context B), and one object which was present in context A on day 2 (i.e., unfamiliar object to context B, Figure 2C).
We calculate the discrimination ratio (DR) on day 3 as a measure for object-in-context recognition memory. The DR is calculated as time spent with the novel object compared to the total exploration time of both objects on day 3 [tnovel/(tnovel + tfamiliar) (Figure 3); Dix and Aggleton, 1999; Kanasou et al., 2015a and 2015b].
Note: It should be tested whether the DR is significantly higher against the chance level (50%) by using a one sample t-test. If not significant, then the animals are not considered to learn.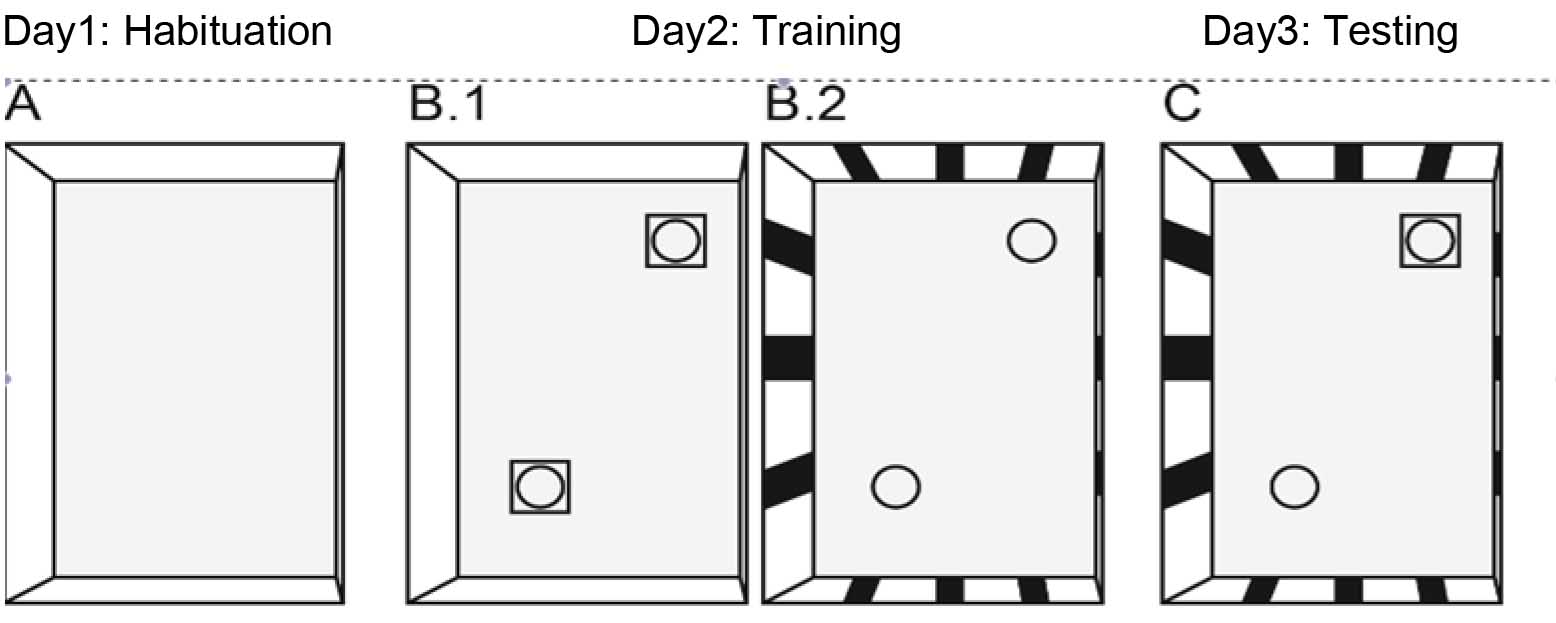
Figure 2. Schematic representation indicating the setup of the object in-context recognition task (Kanatsou et al., 2015a; Kanatsou et al., 2015b). A. Mice are initially habituated in a context that had no object. B1. During training, mice are placed in the same context but with two identical objects and then placed in a novel context with two identical novel objects B2. C. Finally the mice are placed in the latter context but with one object being replaced by an object from the first context. - Analysis
Sniffing is scored as object-exploration behavior if the mouse displayed such behavior towards an object within a distance of 2 cm maximum from the object. Climbing on top of or “watching” the objects from a (close) distance is not considered as sniffing behavior. The exploration time mice are sniffing the objects, is recorded manually by two independent researchers.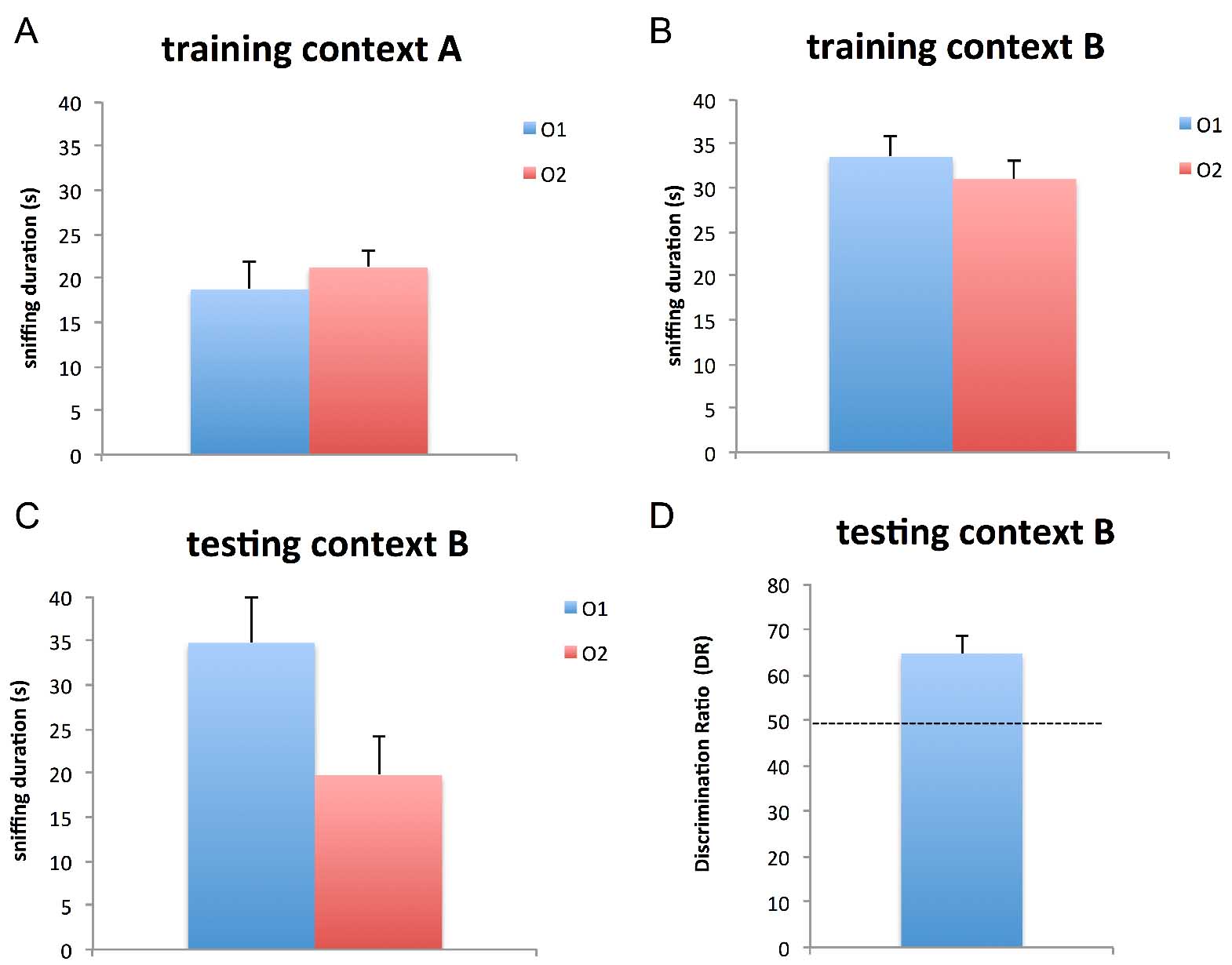
Figure 3. OIC memory in male mice. A. At day 2, mice showed no preference for the objects in context A, as they spent equal amounts of time (around 20 sec) exploring the two identical objects. B. At day 2, mice spent equal amounts of time (around 30 sec) exploring the two similar objects in context B. C. At day 3, mice spent more time sniffing the object O1 (new object; from context A) compared to the familiar object O2. D. At day 3, mice show higher preference (~60%) for the novel object in the new context [O1 from context A in the new context (context B)]. n = 4 animals, error bars represent standard error of the mean.
Notes
- All objects should be cleaned thoroughly between tests, and placed at a 15 cm distance from the corners of the box (glass bottle 9.5 cm high and the Lego block 8.0 cm high).
- About half of the old bedding material is removed and fresh bedding material is added on top of the old material and mixed thoroughly in between each session, to saturate the olfactory cues of previous mice tested.
- All mice are introduced in the contexts by facing the same wall.
- Before starting testing, add a little bit of used bedding material (e.g., from the home cage) to each context and mix very well with the new bedding in each context. That way you rule out the possibility that the odor cues from previous mouse tested may confound the result.
- The light intensity should be equal throughout the box (approximately 30 lux). Unequal light intensity (especially in the objects) can cause a preference for one of the objects.
- At the end of each day, throw away all the bedding and clean the walls of all boxes with 70% ethanol.
- Mice are not habituated in the behavior room before testing.
Acknowledgments
This protocol was adapted from the previous works Dix and Aggleton (1999) and Barsegyan et al. (2014). SFK was supported by ALW grant # 821-02-007 from the Netherlands Organization for Scientific Research NWO.
References
- Albasser, M. M., Davies, M., Futter, J. E. and Aggleton, J. P. (2009). Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci 123(1): 115-124.
- Balderas, I., Rodriguez-Ortiz, C. J., Salgado-Tonda, P., Chavez-Hurtado, J., McGaugh, J. L. and Bermudez-Rattoni, F. (2008). The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem 15(9): 618-624.
- Banks, P. J., Warburton, E. C., Brown, M. W. and Bashir, Z. I. (2014). Mechanisms of synaptic plasticity and recognition memory in the perirhinal cortex. Prog Mol Biol Transl Sci 122: 193-209.
- Barsegyan, A., McGaugh, J. L. and Roozendaal, B. (2014). Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Front Behav Neurosci 8: 160.
- Bermudez-Rattoni, F. (2014). The forgotten insular cortex: its role on recognition memory formation. Neurobiol Learn Mem 109: 207-216.
- Bermudez-Rattoni, F., Okuda, S., Roozendaal, B. and McGaugh, J. L. (2005). Insular cortex is involved in consolidation of object recognition memory. Learn Mem 12(5): 447-449.
- Bussey, T. J., Duck, J., Muir, J. L. and Aggleton, J. P. (2000). Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res 111(1-2): 187-202.
- Bussey, T. J., Muir, J. L. and Aggleton, J. P. (1999). Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci 19(1): 495-502.
- Dix, S. L. and Aggleton, J. P. (1999). Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res 99(2): 191-200.
- Eacott, M. J. and Norman, G. (2004). Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci 24(8): 1948-1953.
- Ennaceur, A. and Aggleton, J. P. (1997). The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res 88(2): 181-193.
- Kanatsou, S., Kuil, L. E., Arp, M., Oitzl, M. S., Harris, A. P., Seckl, J. R., Krugers, H. J. and Joels, M. (2015a). Overexpression of mineralocorticoid receptors does not affect memory and anxiety-like behavior in female mice. Front Behav Neurosci 9: 182.
- Kanatsou, S., Fearey, B. C., Kuil, L. E., Lucassen, P. J., Harris, A. P., Seckl, J. R., Krugers, H. and Joels, M. (2015b). Overexpression of mineralocorticoid receptors partially prevents chronic stress-induced reductions in hippocampal memory and structural plasticity. PLoS One 10(11): e0142012.
- Roozendaal, B., Hernandez, A., Cabrera, S. M., Hagewoud, R., Malvaez, M., Stefanko, D. P., Haettig, J. and Wood, M. A. (2010). Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30(14): 5037-5046.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kanatsou, S. and Krugers, H. (2016). Object-context Recognition Memory Test for Mice. Bio-protocol 6(17): e1925. DOI: 10.21769/BioProtoc.1925.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








