- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Joint-infiltrating Cells
Published: Vol 6, Iss 17, Sep 5, 2016 DOI: 10.21769/BioProtoc.1911 Views: 11369
Reviewed by: Ivan ZanoniAchille BroggiMarco Di Gioia

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selection of Molecules with Immunological Potential from Excretory and Secretory Products from the Nematode Haemonchus placei by Cell Proliferation and Gene Expression Assays
Jocelyn Maza-Lopez [...] Carla O. Contreras-Ochoa
Jun 20, 2023 1331 Views

Primary Mouse Invariant Natural Killer T (iNKT) Cell Purification and Transduction
Gloria Delfanti [...] Giulia Casorati
Jul 5, 2023 2172 Views

Nuclei Isolation Methods on Frozen Clotted Blood Samples
Melissa Cuevas [...] Nancy Hadley Miller
Jan 20, 2026 334 Views
Abstract
Infiltration of leukocytes into joints is one of the main features of autoimmune inflammatory arthritis. Here, we describe the protocol for isolation of joint-infiltrating cells in mice. This protocol is useful to analyze cell surface antigens and intracellular cytokines by flow cytometry.
Keywords: JointMaterials and Reagents
- 27-gauge needle (TERUMO, catalog number: NN-2719S )
- 1 ml syringe (TERUMO, catalog number: SS-01T )
- 6-well plate (Coring, Falcon®, catalog number: 353046 )
- 50 ml tube
- 70 μm cell strainer (Coring, Falcon®, catalog number: 352350 )
- 10 ml syringe (TERUMO, catalog number: SS-10SZ )
- Mice (adult > 6 weeks, any sex, any strain)
- Hyaluronidase (60 mg/ml stock) (Sigma-Aldrich, catalog number: H3506 )
- Collagenase type VIII (10 mg/ml stock) (Sigma-Aldrich, catalog number: C2139 )
- RPMI 1640 (Sigma-Aldrich, catalog number: R8758 )
- Fetal bovine serum, heat inactivated (Biowest, catalog number: S1820-500 )
- HANK’s solution “Nissui”② (NISSUI PHARMACEUTICAL, catalog number: 05906 )
- NaHCO3 (NAKARAI TESQUE, catalog number: 31212-25 )
- NaN3 (NAKARAI TESQUE, catalog number: 31208-82 )
- Digestion medium (see Recipes)
- FACS solution (see Recipes)
Equipment
- Surgical scissors and tweezers
- Mini-shaker 3D (Biosan, catalog number: BS-010151-AAK )
- Refrigerated centrifuge
Procedure
- Euthanize mice. Sterilize mouse skin with 70% ethanol and peel off the skin of ankles using scissors and tweezers (Figure 1). Be careful not to leave any skin.
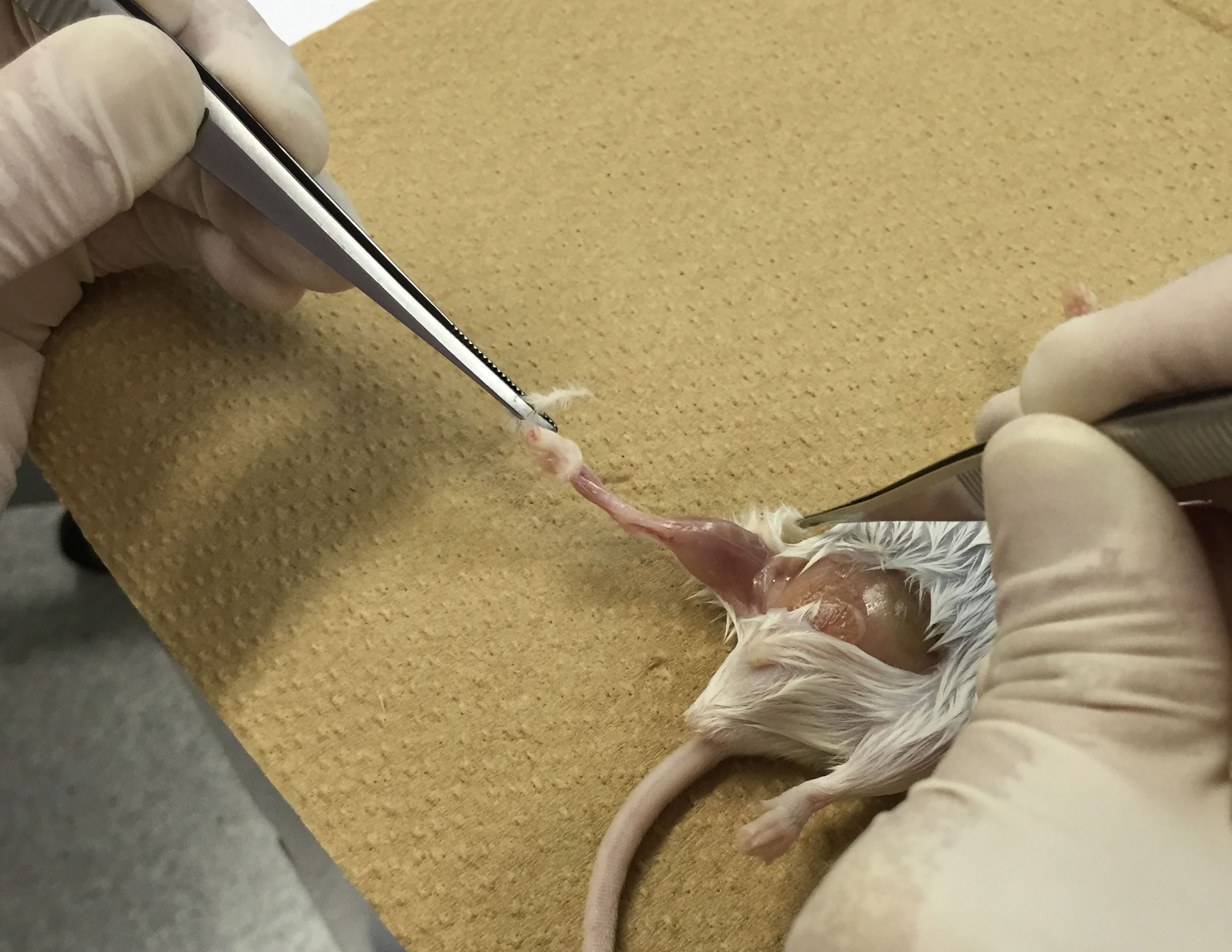
Figure 1. Peel off the skin of ankles
- Cut out the leg at 0.7 cm above the ankle joint using scissors, and discard the finger portion (Figure 2).
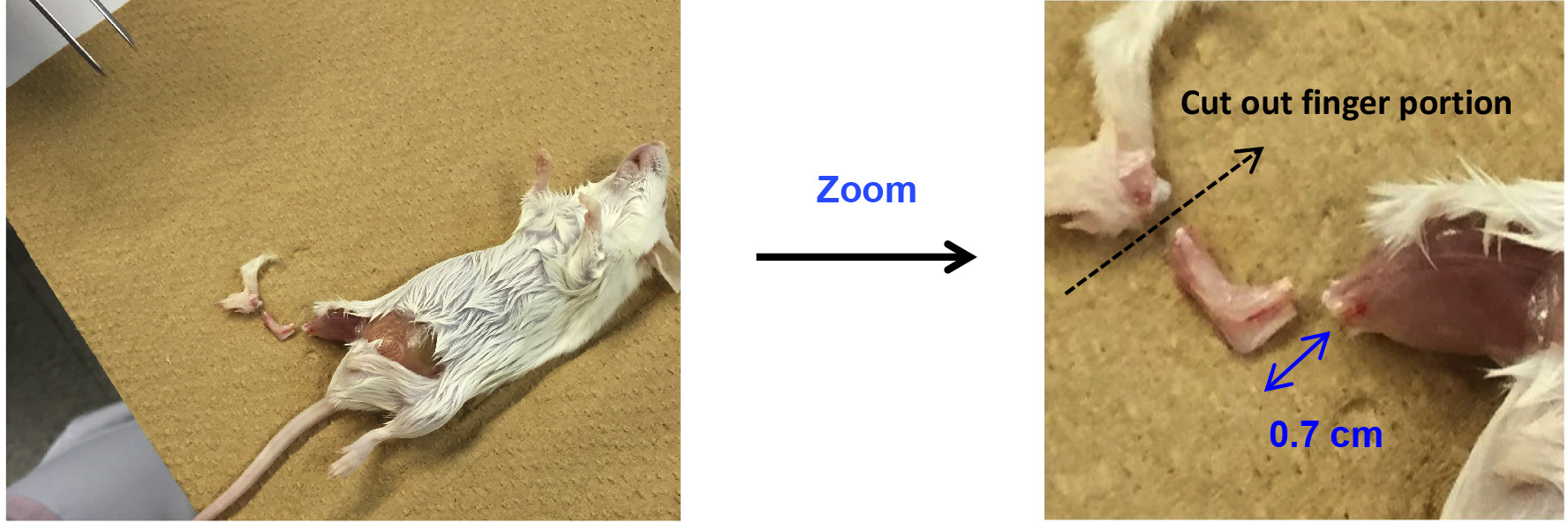
Figure 2. Cut out ankles
- Wash ankle portion in 2 ml RPMI 1640.
- Flush out bone marrow cells using a 27-gauge needle and 1 ml syringe filled with RPMI 1640 and discard the bone marrow cells.
- Put bone marrow-removed ankles in 4 ml of digestion medium on a 6-well plate (Figure 3).
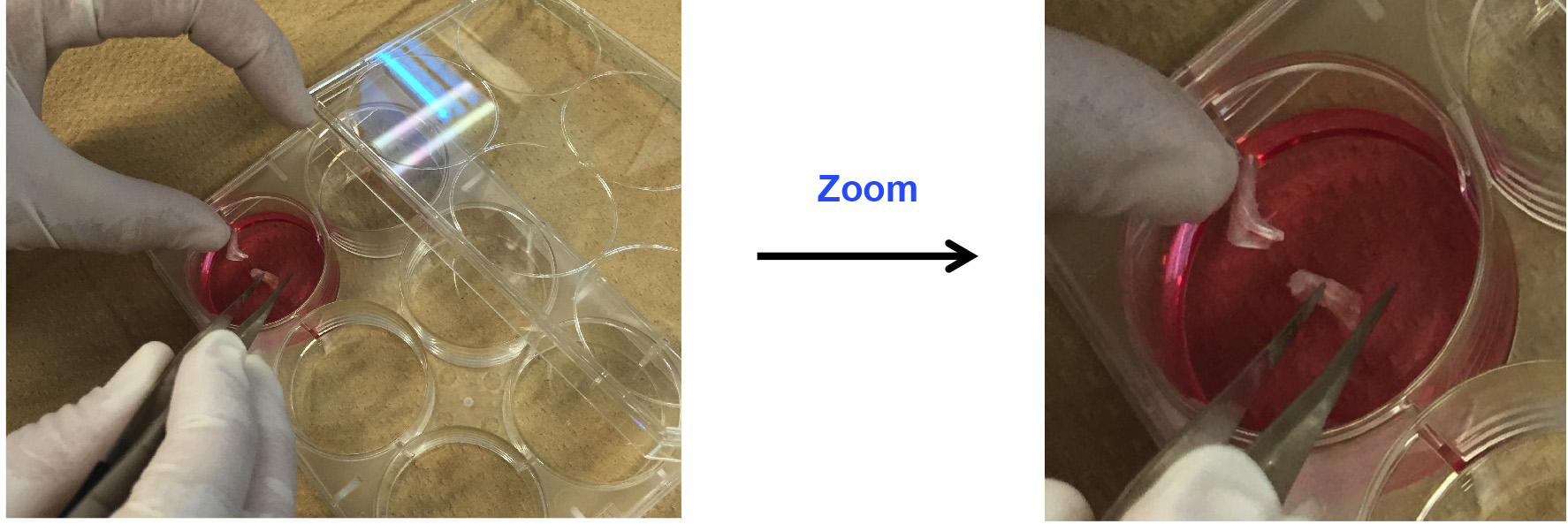
Figure 3. Put ankles in digestion medium - Chop up ankles with scissors to 3-4 mm sized chunks (Figure 4).
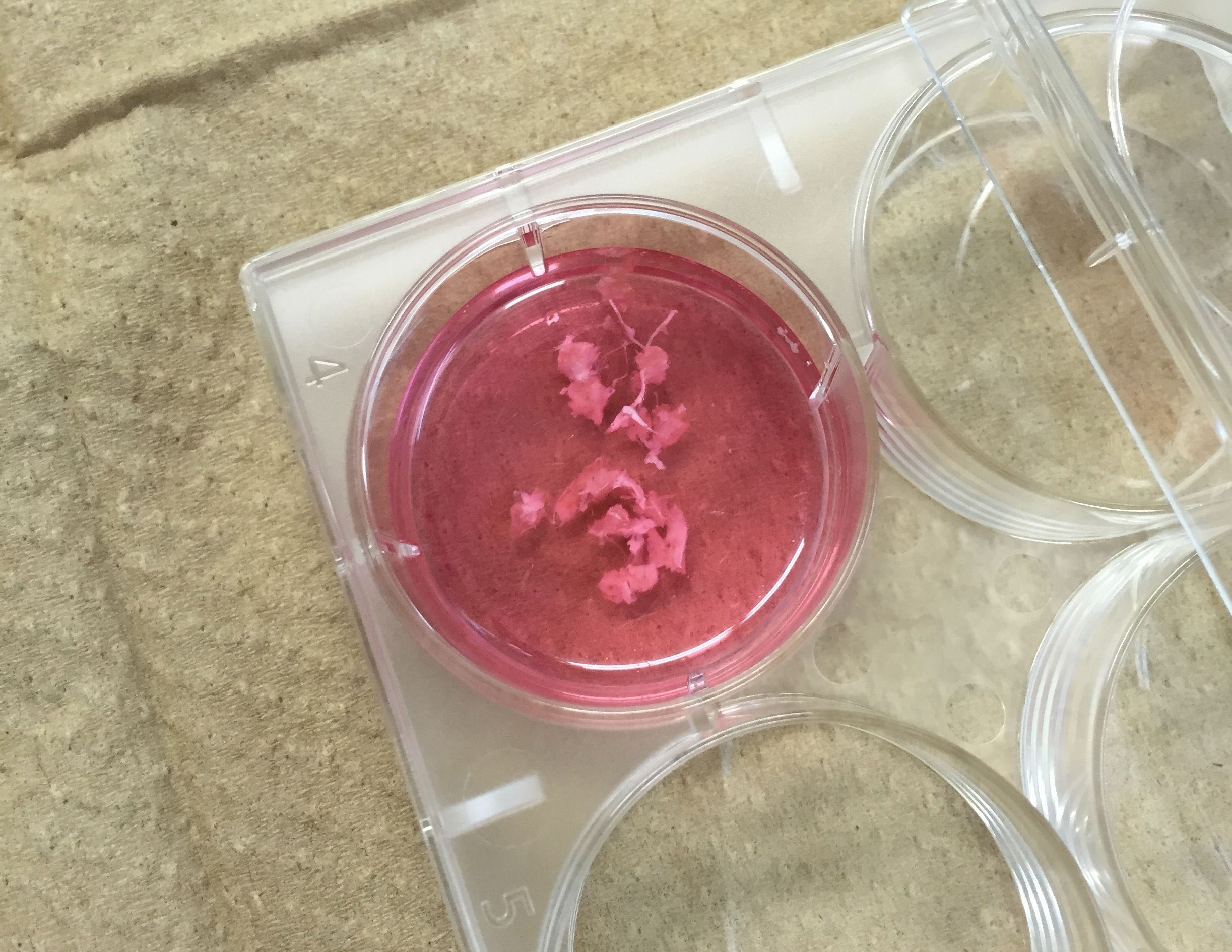
Figure 4. Chop up ankles
- Incubate ankles in a 6-well plate with gentle shaking by Mini-shaker for 1 h at 37 °C under 5% CO2.
- Place a cell strainer in a 50 ml tube. Then, transfer the medium with digested ankle pieces onto the cell strainer.
- Remove the plunger from a 10 ml syringe. Mash the residues of ankle pieces using the black rubber end of the plunger.
- Add 10 ml 10% FBS/RPMI 1640.
- Centrifuge at 1,500 rpm (400 x g) for 5 min at 4 °C.
- Discard the supernatant and resuspend the cell pellet in FACS solution.
- Count viable cells. Representative data are shown below (Figure 5).
- Perform FACS analysis. Representative data are shown below (Figure 6).
Representative data
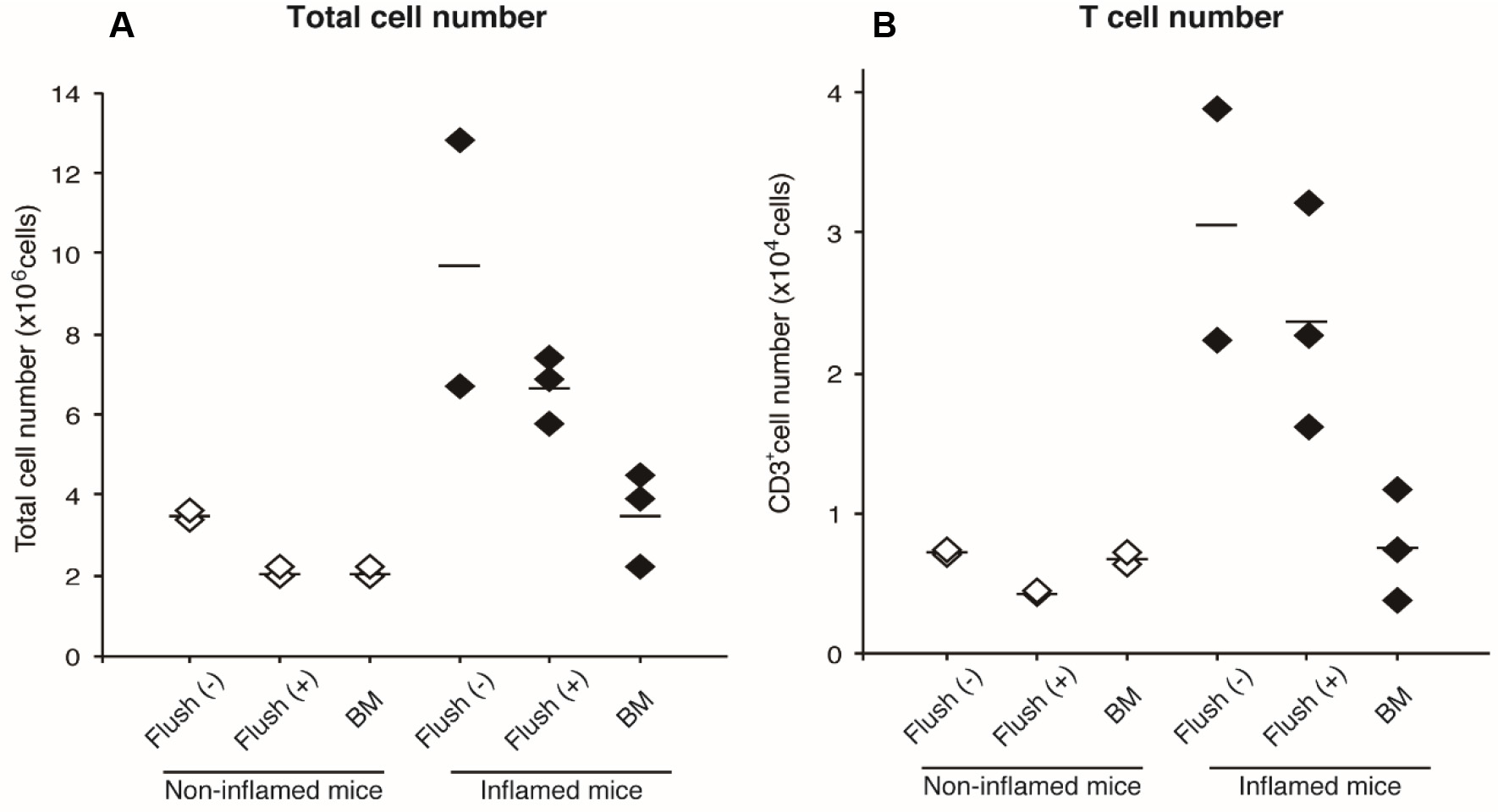
Figure 5. Cell numbers in ankle joints. Total cell numbers (A) and T cell numbers (B) obtained by this protocol are shown. White diamonds represent cells from non-inflamed ankles in a WT mouse, and black diamonds represent cells from inflamed ankles in an Il1rn-/- mouse (Akitsu et al., 2015). Each symbol indicates number of joint cells without flushing (step 4) [Flush (-)], bone marrow-removed joint cells [(Flush (+)], and bone marrow cells (BM). Horizontal lines indicate medians.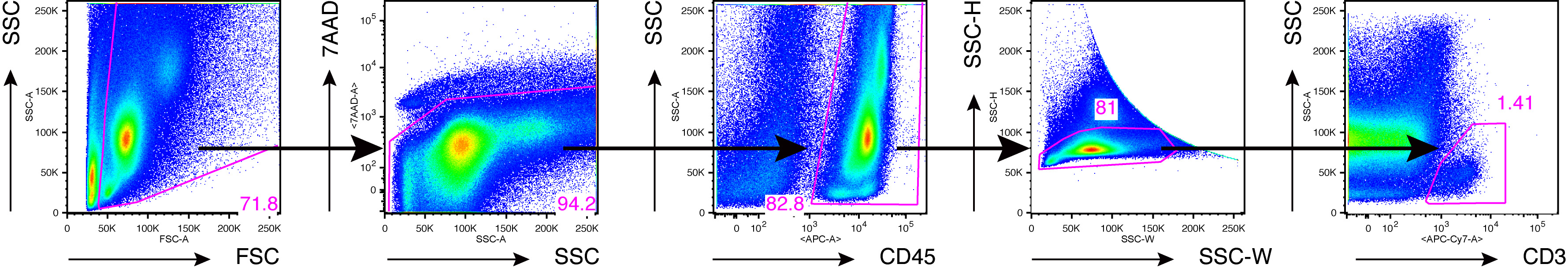
Figure 6. An example of FACS analysis of joint-infiltrated cells from inflamed ankles. Flow cytometry of joint-infiltrating cells from Il1rn-/- mice (Akitsu et al., 2015). Numbers indicate percent cells in each gate. Data are representative of 10 independent experiments.
Notes
- Similar data were obtained by ten independent experiments using this protocol.
- If joints are arthritic with inflammation, joint cells from a mouse are enough to analyze with FACS. However, for normal joints, a pool of two to four mice is recommended.
- From arthritic mice, 2-4 x 104 T cells are obtained from one mouse (without flushing step). Because bone marrow-derived cells are about 1/5 of the total T cells, flushing step can be skipped. On the other hand, total cell numbers without flushing step are approximately 4 x 106 cells/non-inflamed ankles, and 7-13 x 106 cells/inflamed ankles. Because 1/2-1/3 of them are derived from bone marrow, flushing step is necessary for analyzing non-T cell population. After the flushing step, total joint-infiltrated cell numbers are approximately 2 x 106 cells in non-inflamed ankles, and 5-7 x 106 cells in inflamed ankles.
Recipes
- Digestion medium
160 μl hyaluronidase (final concentration: 2.4 mg/ml)
400 μl collagenase VIII (final concentration: 1 mg/ml)
4 ml of RPMI 1640 supplemented with 10% FBS - FACS solution
Dissolve 4.9 g HANK’s in 490 ml H2O
Add 0.15 g NaHCO3 and 0.05 g NaN3, and dissolve completely
Add 10 ml inactivated FBS (final 2%), then mix
Store at 4 °C until use
Acknowledgments
This work was supported by a Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Akitsu, A., Ishigame, H., Kakuta, S., Chung, S. H., Ikeda, S., Shimizu, K., Kubo, S., Liu, Y., Umemura, M., Matsuzaki, G., Yoshikai, Y., Saijo, S. and Iwakura, Y. (2015). IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vγ6(+)γδ T cells. Nat Commun 6: 7464.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Akitsu, A. and Iwakura, Y. (2016). Isolation of Joint-infiltrating Cells. Bio-protocol 6(17): e1911. DOI: 10.21769/BioProtoc.1911.
Category
Immunology > Immune cell isolation > Leukocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











