- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Bone Marrow-derived Endothelial Progenitor Cell Intercellular Adhesion Assay
Published: Vol 6, Iss 16, Aug 20, 2016 DOI: 10.21769/BioProtoc.1909 Views: 10957
Reviewed by: Xiujun FanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Isolation of CD31+ Bone Marrow Endothelial Cells (BMECs) from Mice
Alhaji Osman Smith [...] Lingyu Zeng
Nov 20, 2021 4072 Views

Static Adhesion Assay for Human Peripheral Blood Mononuclear Cells
Giulia Vanoni [...] Sara Trabanelli
Jan 5, 2022 3816 Views

An Unbiased CRISPR-Cas9 Screening Method for the Identification of Positive and Negative Regulatory Proteins of Cell Adhesion
Yvonne J. Thus [...] Marcel Spaargaren
Nov 5, 2022 2793 Views
Abstract
Cell-cell adhesion ensures tight contacts between neighbouring cells, which is necessary for cell segregation, as well as for the morphological and functional differentiation of different tissues. Evidently there are cell-cell recognition systems that make cells of the same type preferentially adherent to one another. Homotypic cell adhesion is particularly important in mediating a range of physiological processes such as cell survival, migration and remodeling of vessels. Thus in the present study we selected two populations of endothelial progenitor cells which are from the same donor to investigate the possible effect of a small molecule compound Icariin on homotypic cell adhesion. Many angiogenic factors can destabilize the organization of intercellular junctions, causing endothelial barrier opening. In the present study, we observed that Icariin treatment reduced the level of VE-cadherin expression in EPCs indicating a decrease in cell-cell adhesion-a proof of the pro-angiogenic effect of Icariin. In summary, the observed loss of homotypic adhesion of EPCs may contribute to the enhanced angiogenic effect exerted by Icariin.
Keywords: Endothelial progenitor cellMaterials and Reagents
- Sterile serological transfer pipettes (Greiner Bio-One, catalog number: 606107 , 607107 , 760107 )
- Sterile centrifuge tubes (Greiner Bio-One, catalog number: 188261 )
- T75 culture flasks (Corning, catalog number: 353135 )
- 24-well plates (Corning, Costar®, catalog number: 3524 )
- 96-well plates (Corning, catalog number: 3596 )
- Human bone marrow-derived endothelial progenitor cells (BM-EPCs) [isolated from bone marrow of healthy donors using Ficoll-Paque density gradient (1.077 g/cm3)] (Tang et al., 2015)
- Dulbecco’s phosphate-buffered saline (DPBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190169 )
- Human fibronectin (BD Biosciences, catalog number: 610077 )
- Endothelial cell growth medium MV2 (ECGM2) (PromoCell GmbH, catalog number: 22121 )
- Penicillin/streptomycin (5,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15070-063 )
- Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- Dil-ac-LDL (Thermo Fisher Scientific, catalog number: L3484 )
- FITC-UEA-I (Sigma-Aldrich, catalog number: L9006 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: 158127 )
- Hoechst 33258 (Santa Cruz Biotechnology, catalog number: sc-394039 )
- Calcein AM (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: L-3224 )
- Ethylene diamine tetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E6758 )
- Trypsin from porcine pancreas (Sigma-Aldrich, catalog number: T4799 )
- Endothelial cell growth medium (see Recipes)
- 0.2 mg/ml Hoechst 33258 (see Recipes)
- 2 μM calcein-AM (see Recipes)
Equipment
- Horizontal centrifuge (Eppendorf, catalog number: 5702RH )
- CO2 cell culture incubator (Thermo Fisher Scientific, catalog number: 51026280 )
- Flow cytometer (LSR II) (BD Biosciences, catalog number: 342975 )
- Scepter 2.0H handheld automated cell counter, w/50 pk of 60 µM Sensors (Merck, Merck Millipore, catalog number: PHCC20060 )
- Biological safety cabinets (Thermo Fisher Scientific, catalog number: 51026639 )
- Inverted fluorescence microscope (Zeiss, catalog number: Apotome.2 )
Procedure
- Bone marrow sample was diluted with equal volume of DPBS, then the diluted sample was carefully layered onto the top of Ficoll-Paque PLUS. Centrifuge at 400 x g for 30 min with the brake off (Figure 1).
- The mononuclear cells were carefully harvested from the interface between the Ficoll-Paque PLUS and plasma using a sterile Pasteur pipette, and transfered to sterile centrifuge tubes.
- Cells were washed with an equal volume of DPBS twice to remove the Ficoll-Paque PLUS residue. After washing, the isolated mononuclear cells were cultivated in flasks coated with human fibronectin (25 μg/ml) and induced by ECGM2 medium at 37 °C with 5% CO2 in humidified air at a density of 3-5 x 106/cm2 (Figure 2).
- After 72 h in culture, non-adherent cells were removed by washing with phosphate-buffered saline (PBS), preheated fresh ECGM2 medium was applied and the cultivation was maintained through 7 d. All cells used in this experiment are cultivated with ECGM2 at passages 3 to 5.
- Quantitative fluorescence-activated cell sorting (FACS) was performed on a vantage SE flow cytometer to detect the surface marker of CD34, CD133, KDR, VE-cadherin, E-selectin, vWF on the cells at 7 and 14 d.
- Immunofluorescence staining was performed using Dil-ac-LDL and FITC-UEA-I. Brifely, cells were first incubated with Dil-ac-LDL (5 μg/ml) at 37 °C for 2 h and then fixed through incubation with 4% paraformaldehyde for 20 min. They were subsequently incubated with UEA (10 μg/ml) at 37 °C for 1 h. After washing with PBS, the cells were visualized and photographed using a fluorescence microscope and counted at 200x magnification. Double-positive cells were considered to be differentiating EPCs.
- Plate 5 x 103 BM-EPCs in a 96-wells plate overnight to a confluent monolayer in ECGM2.
- Label the cells with 10 μg/ml Hoechst 33258 dye in PBS for 30 min at 37 °C, followed by washing with PBS three times. After washing, the cells are kept in the PBS in the cell incubator until the inoculating of another set of cells.
- Another set of BM-EPCs from the same donor are starved in a 96-wells plate (5 x 103/well) with basic medium (without any supplemented growth factors or fetal bovine serum) for 24 h, and treated with small molecule compounds.
- After 48 h incubation with the compounds to be investigated, the cells are washed twice with PBS, incubated with 2 μM calcein AM (100 μl/well) for 1 h in the cell incubator at 37 °C, followed by washing with PBS three times.
- The cells are detached using 0.25% trypsin-0.02% EDTA (50 μl/well) for 3 min, then resuspended with 100 μl ECGM2 and plated onto the established cell monolayer (acceptor cells) with the density of 3 x 103/well.
- The cultures were incubated in the PBS for 20 min at 37 °C in a 5% CO2 cell culture incubator.
- After that the cultures are washed gently using PBS (100 μl/well) three times.
- Attachment and spreading of the plated cells are monitored and recorded with a fluorescence microscope every 5 min (Figure 3).
- Quantification of intercellular adhesion is performed by counting the number of cells that are calcein-AM positive per microscopic field of view.
Representative data
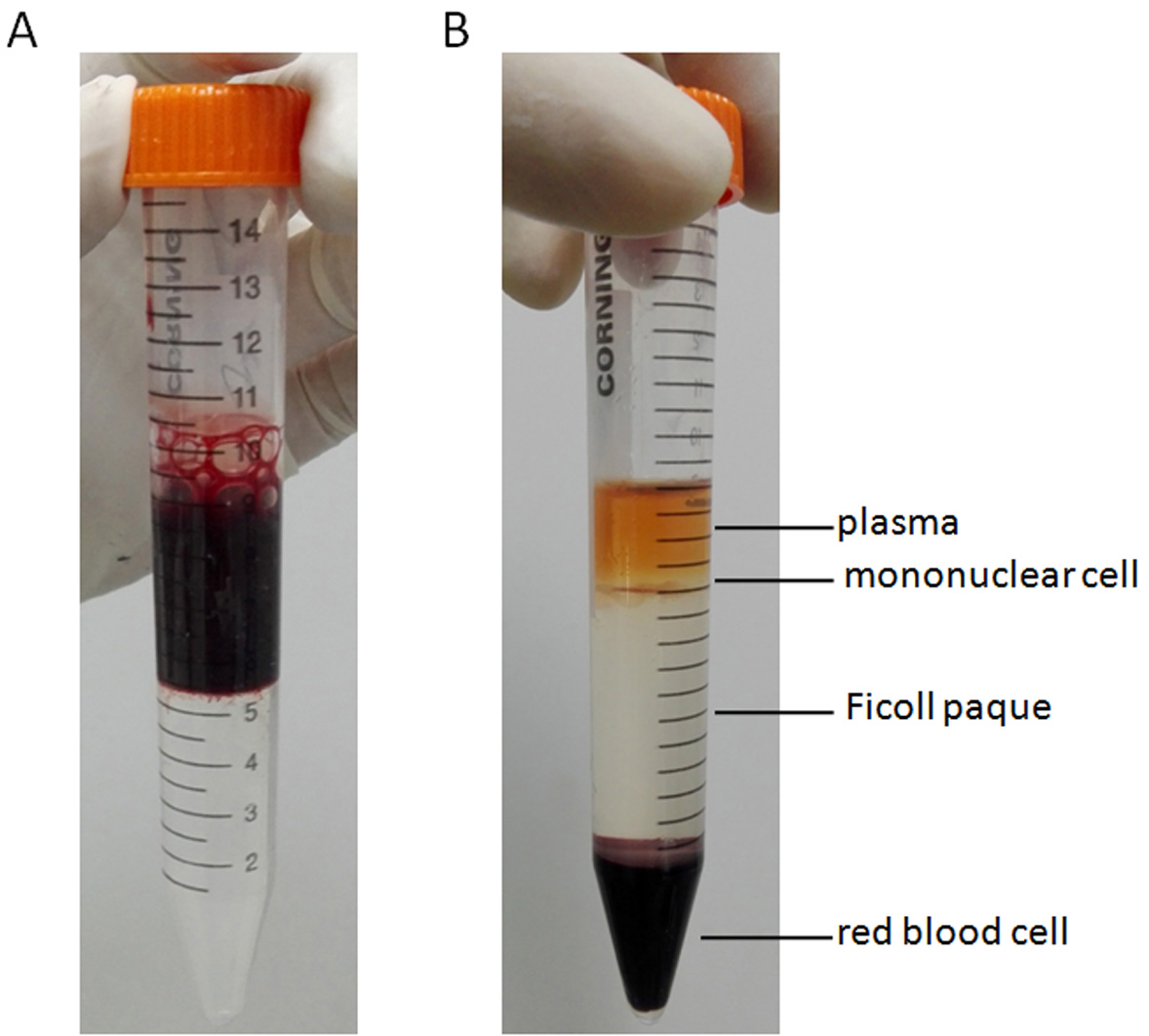
Figure 1. Images of cells collected from the interface between the Ficoll-Paque PLUS and plasma. A. Bone marrow sample was diluted with equal volume of DPBS, then the diluted sample was carefully layered onto the top of Ficoll-Paque PLUS. B. After centrifuging at 400 x g for 30 min, the mononuclear cells were harvested from the interface between the Ficoll-Paque PLUS and plasma.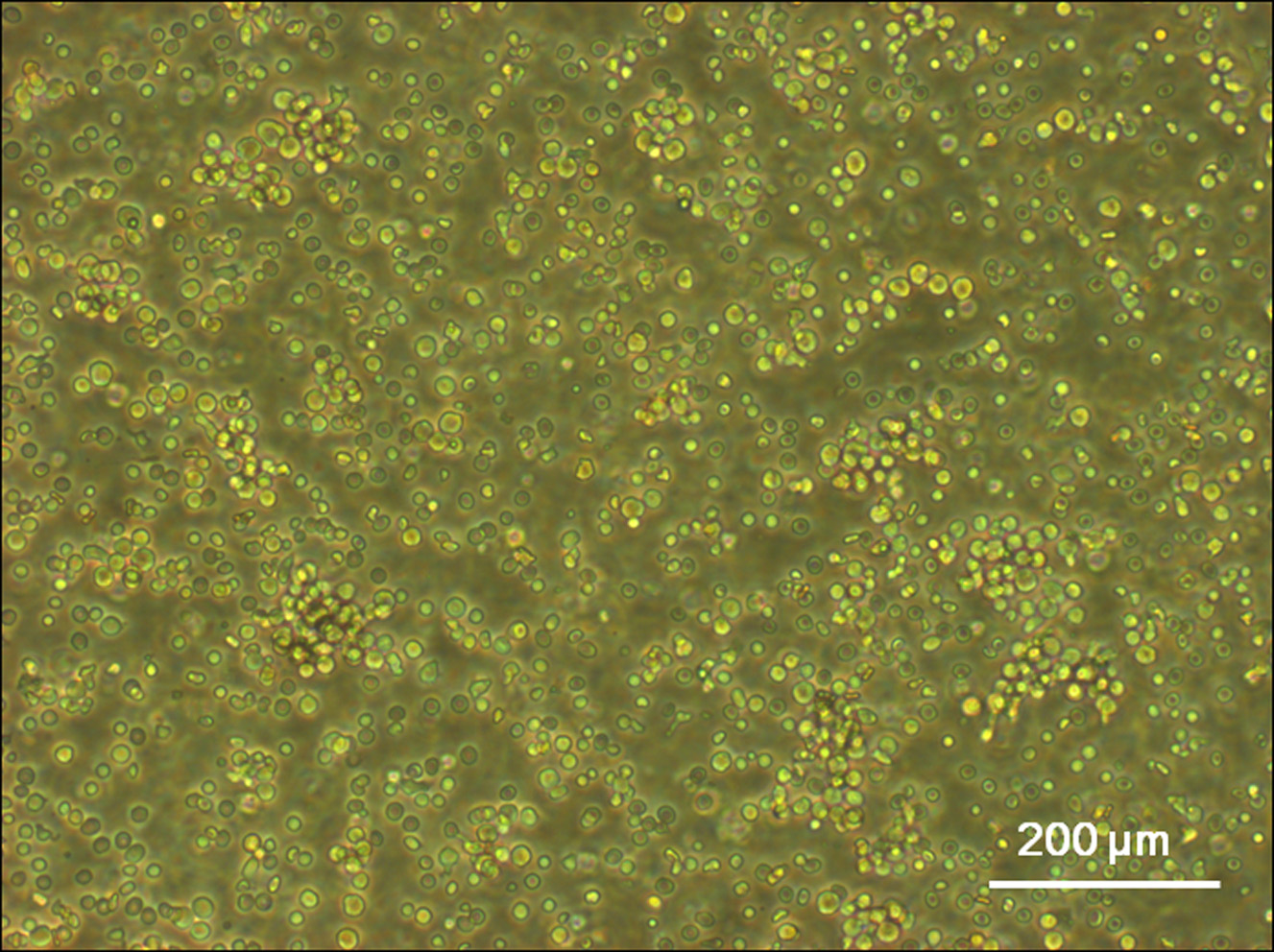
Figure 2. Light microscope image of mononuclear cells. The mononuclear cells were carefully harvested from the interface between the Ficoll-Paque PLUS and plasma using a sterile Pasteur pipette, and transfered to sterile centrifuge tubes. After washing, the isolated mononuclear cells were cultivated in flasks coated with human fibronectin (25 μg/ml) and induced by ECGM2 medium at 37 °C with 5% CO2 in humidified air at a density of 3-5 x 106/cm2.
Figure 3. Fluorescence microscope images of cells after intercellular adhesion assay. A. Cells were plated overnight to a confluent monolayer in ECGM2, and labeled the cells with Hoechst 33258. B. Representative fluorescence micrograph demonstrating cells after intercellular adhesion assay.
Notes
- This protocol can be used for other cell lines, however we recommend a pilot experiment to find optimal conditions for any cell line used. These include:
- Optimal cell density, which is dictated by several parameters such as cell size (the larger the cells, the lower the density) and cell adhesive ability.
- Time of attachment. Weaker adherent cell lines may require longer incubation time to track adhesive formation.
Recipes
- Endothelial cell growth medium
0.02 ml/ml fetal calf serum
5 ng/ml epidermal growth factor (recombinant human)
10 ng/ml basic fibroblast growth factor (recombinant human)
20 ng/ml insulin-like growth factor (Long R3 IGF)
0.5 ng/ml vascular endothelial growth factor 165 (recombinant human)
1 μg/ml ascorbic acid
22.5 μg/ml heparin
0.2 μg/ml hydrocortisone
100 U/ml of penicillin/streptomycin - 0.2 mg/ml Hoechst 33258
10 mg Hoechst 33258
50 ml of sterile PBS
Keep this stock solution at 4 °C in shelters from light.
Note: The stock solution can only be kept for 30 d. - 2 μM calcein-AM
Add 5 µl of the supplied 4 mM calcein-AM stock solution to 10 ml of sterile, tissue culture-grade D-PBS, vortexing to ensure thorough mixing. This gives an approximately 2 µM calcein-AM solution.
Note: This staining solution should be freshly prepared each time. The stock solution must be kept at -20 °C and protected from light.
Acknowledgments
The concept of this protocol was adapted from our previous study, in which the intercellular adhesion assay was used to measure cell-cell adhesion ability of endothelial progenitor cells (Tang et al., 2015).This study was supported by the German Academic Exchange Service and Federal Ministry of Education and Research (D/09/04774).
References
- Tang, Y., Jacobi, A., Vater, C., Zou, L., Zou, X. and Stiehler, M. (2015). Icariin promotes angiogenic differentiation and prevents oxidative stress-induced autophagy in endothelial progenitor cells. Stem Cells 33(6): 1863-1877.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tang, Y., Jacobi, A., Vater, C., Zou, X. and Stiehler, M. (2016). Bone Marrow-derived Endothelial Progenitor Cell Intercellular Adhesion Assay. Bio-protocol 6(16): e1909. DOI: 10.21769/BioProtoc.1909.
Category
Stem Cell > Adult stem cell > Endothelial stem/progenitor cell
Cell Biology > Cell movement > Cell adhesion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link