- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Controlled Cortical Impact Mouse Model for Mild Traumatic Brain Injury
Published: Vol 6, Iss 16, Aug 20, 2016 DOI: 10.21769/BioProtoc.1901 Views: 13724
Reviewed by: Soyun KimXiaoyu LiuXi Feng

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for Measuring Free (Low-stress) Exploration in Rats
Wojciech Pisula and Klaudia Modlinska
Jan 20, 2020 4459 Views

Operant Vapor Self-administration in Mice
Renata C. N. Marchette [...] Khaled Moussawi
May 20, 2021 4569 Views

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1772 Views
Abstract
Traumatic brain injury (TBI) affects millions of people worldwide; however, the immediate impact of TBI and the secondary injury mechanisms are still not fully understood. TBI can cause devastating neuromotor deficits in both acute and chronic stages. Time course studies utilizing animal models of focal TBI have provided essential insight into TBI neuropathology. Here, we describe a surgical technique for creating a mouse model of focal, mild TBI (Dixon et al., 1991; Smith et al., 1995; Bolkvadze and Pitkanen, 2012). Furthermore, we provide protocols for validating TBI models using behavioral tests that examine post-traumatic neuromotor deficits resulting from TBI neuropathology (Fujimoto et al., 2004; Febinger et al., 2015; Smith et al., 1995; Bolkvadze and Pitkanen, 2012).
Keywords: TBIMaterials and Reagents
- Sterile cotton tipped applicators
- Gauze pads (ATC Medical, CurityTM, catalog number: 686132 )
- Nolvasan surgical scrub (Zoetis Inc., catalog number: 8NOL413 )
- Adult C57BL6/J laboratory mice (6 weeks old)
- 70% ethanol
- Isoflurane
- Opthalmic ointment
- 3.0% hydrogen peroxide solution
- Sterile saline
- VetBond glue (3M, catalog number: 1469SB )
- 70% isopropanol
Equipment
- Surgical Tools
- Scalpel handle (Fine Science Tools, catalog number: 10003-12 )
- Sterile scalpel blades (Fine Science Tools, catalog number: 10010-00 )
- Dumont forceps (Fine Science Tools, catalog number: 11251-10 )
- 5.0-mm diameter trephine (Figure 1) (Fine Science Tools, catalog number: 18004-50 )
- Pin vise (Figure 1) (ProEdge, catalog number: PRO58111 )

Figure 1. Trephine in pin vise - Hot bead sterilizer (Fine Science Tools, catalog number: 18000-45 )
- Warm water recirculator (Kent Scientific, catalog number: TP-700 )
- Polystyrene weighing boat
- Controlled cortical impact (CCI) device (Impact One Stereotaxic Impactor for CCI) (Leica Biosystems, catalog number: 39463920 )
- 3.0-mm diameter metal piston (Leica Biosystems , catalog number: 39463920)
- Small animal stereotaxic instrument with mouse adapters (Kopf Instruments, model: 900 and 923-B )
- Scale
- Leather hole punch, sharp (Anytime Tools)
- Incandescent lamp (50-75 watt)
- Inclined plane device, custom made (Figure 2) (The angle board device is 50 x 60 cm in size and is covered with a vertically grooved rubber mat. The platform was attached to a hinge, allowing the researcher to manually adjust the angle of the board.)
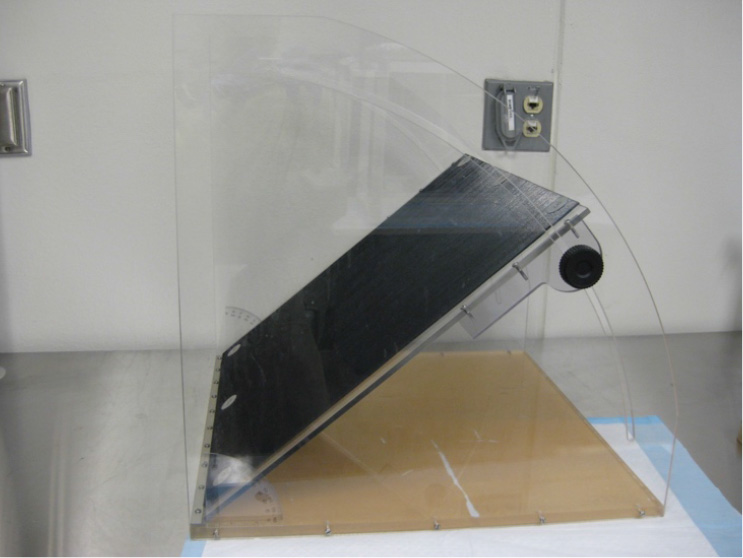
Figure 2. Inclined plane (custom made)
Procedure
- Animal preparation
- Autoclave surgical tools, cotton tipped applicators, and gauze pads. Disinfect the polystyrene weighing boat with 70% ethanol.
- Prepare the surgical area. Turn on the warm water recirculator, and the hot bead sterilizer so that they can warm up. It is recommended to bead sterilize each surgical tool before use. Be sure to cool the tool before using it on the mouse.
- Impactor device should be placed near surgical area. Screw in 3.0-mm diameter metal piston and set the impact velocity to 5.0 m/s and dwell time of 100 ms from the dura for a mild injury (Figure 3).

Figure 3. Stereotaxic setup
- Impactor device should be placed near surgical area. Screw in 3.0-mm diameter metal piston and set the impact velocity to 5.0 m/s and dwell time of 100 ms from the dura for a mild injury (Figure 3).
- Weigh the animal and record its mass in grams.
Note: Recording the mouse’s body weight prior to performing surgery is important for monitoring post-operative behavior. - Anesthesia (Figure 4): Place the mouse in the induction box and turn on the oxygen tank so that it is flowing at approximately 1.0 L per minute. Turn on the isoflurane vaporizer to 4.0%. Be sure to monitor the mouse as it is becoming unconscious. After approximately 2-3 min, the animal should exhibit slow, even, quiet breathing. To determine if the mouse is fully anesthetized, pinch the mouse’s toe. If the mouse draws its paw towards its body, it is not appropriately anesthetized. If there is no reaction, turn down the isoflurane to 2.0%-2.5%, and route the isoflurane to the stereotaxic device.

Figure 4. Isoflurane vaporizer and induction box - Stereotaxic device: Place the mouse’s mouth on the bite bar with the teeth in the hole, and move the anesthesia cone over the animal’s nose for a snug fit. Guide the two ear bars into the auditory canals and secure. Use the scales to ensure both ear bars are equally placed. The head should be completely secure and level so that it will not move during the craniotomy. At this time, place ophthalmic ointment generously onto the eyes. To maintain the mouse’s body temperature, a heat source needs to be provided. Placing the mouse on a pad that is temperature controlled with a warm water recirculator is recommended.
- Continue to monitor the depth of anesthesia, and adjust the vaporizer as needed. 1.25%-1.75% should be the proper range of maintenance anesthesia for most mice.
- Remove fur from the mouse’s head using clippers to expose the skin.
- Prepare the surgical site by cleaning the head area with Nolvasan surgical scrub using sterile cotton tipped applicators.
- Surgical procedure
- Using a scalpel blade, make a midline skin incision.
- Using the Dumont forceps, remove as much fascia as possible. Then, apply 3.0% hydrogen peroxide to the skull to clean and dry the skull area.
Note: Be sure to flush the hydrogen peroxide with sterile saline when done. - Once the skull has been dried, create a 5-mm diameter craniotomy over the left parietal cortex (between lambda and bregma) using the 5-mm diameter trephine. Apply gentle, uniform pressure when using the trephine. Once the area feels loose, carefully remove the fragment using Dumont forceps.
- At this point, flush the area with saline to remove any bone fragments that may have accumulated. Be sure to keep the brain moist throughout the surgery. Check the integrity of the dura (Figure 5). If the dura is damaged, it will appear as a detached, light layer. Damaging the dura can confound studies and potentially affect behavior test outcomes.
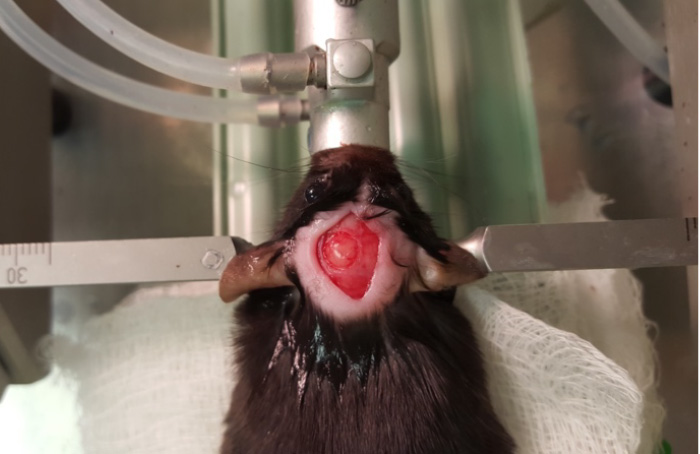
Figure 5. Craniotomy - Attach the controlled cortical impact (CCI) device to the stereotax. Attach the contact sensor to one of the ear bars. Lower the piston until it just touches the dura (the machine will beep when contact is made). Retract the piston and then move down the CCI device 0.5 mm for a mild injury. Then, hit the impact switch on the CCI device (Figures 6 and 7).
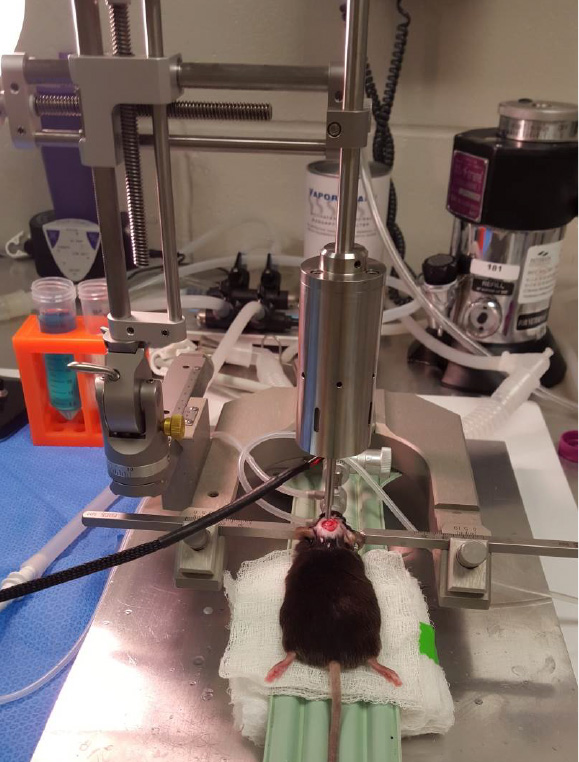
Figure 6. CCI impact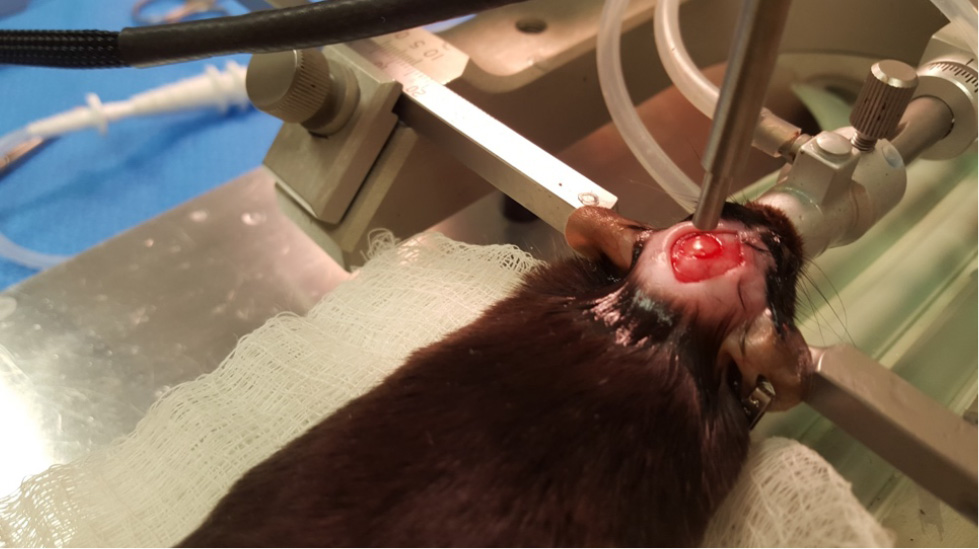
Figure 7. CCI impact - After the impact, flush the area again with sterile saline, and stop any bleeding that may have occurred by applying light pressure with a sterile cotton tipped applicator.
- Then, cover the exposed brain with a 5-mm disc created from a polystyrene weighing boat using the leather hold punch. Apply a small amount of VetBond glue along the edges of the disc. The trick is to keep the brain area moist, but to dry the skull area as much as possible. This will fix the plastic disc to the skull but not the brain (Figure 8). Make sure to remove any VetBond that has adhered to the skin.
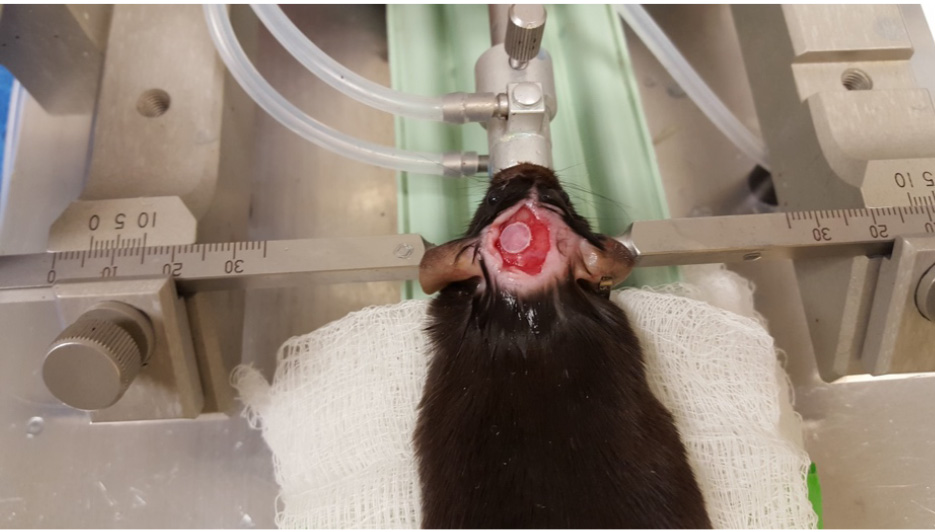
Figure 8. Covering the site of impact - Once the plastic disc is fixed over the injury site, close the incision area with sutures.
- Turn off the isoflurane, and administer analgesics subcutaneously. Remove the mouse from the stereotactic frame, and place the animal in a clean cage. Position an incandescent lamp (50-75 watt) approximately 12 inches away from the animal. Be sure to place the lamp so that only half of the cage is illuminated and the rodent can escape the light if desired. Be sure to monitor the rodent until it has fully woken up and is ambulatory.
- Composite neuroscore behavioral tasks
The composite neuroscore test (CN) was implemented to evaluate posttraumatic neurological deficits using a 28-point system (Fujimoto et al., 2004). For each task, animals are scored on a scale of 0-4, with 0 valued at severely impaired and 4 at no impairment (Fujimoto et al., 2004). Mice undergo a battery of tests: right and left forelimb and hindlimb flexion, right and left lateral pulsion, and inclined plane test. The scores for these tests are added together to calculate the CN.- Hindlimb and forelimb flexion
- Grasp the mouse at the base of the tail while the forepaws remain on a flat surface.
- Observe the hindlimb position for 10 sec. An uninjured animal will characteristically extend its hindlimbs back and out with full extension and spread toes, whereas an injured animal will retract its hindlimbs towards the abdomen and curl its toes (Figures 9 and 10) (Fujimoto et al., 2004). Scoring should be as follows for the left and right limb:
- 4 (no impairment)-Hindlimbs splayed outward, away from the animal's body and toes are spread.
- 3-If the hindlimb briefly retracts towards the abdomen less than 25% of the time, and toes are spread.
- 2-If the hindlimb retracts towards the abdomen more than 25% of the time, but less than 50% of the time, and toes are curled.
- 1-If the hindlimb retracts towards the abdomen more than 50% of the time, and toes are curled.
- 0 (severely impaired)-If the hindlimb is entirely retracted while touching the abdomen and toes are curled for the entire time while lifted.
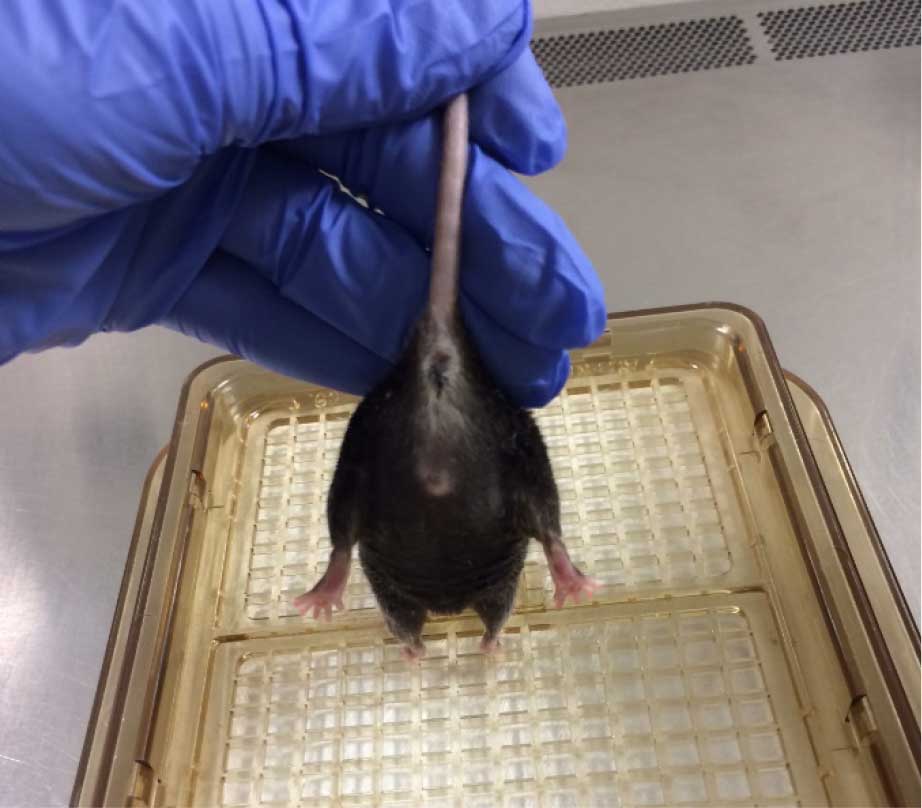
Figure 9. Healthy hindlimb position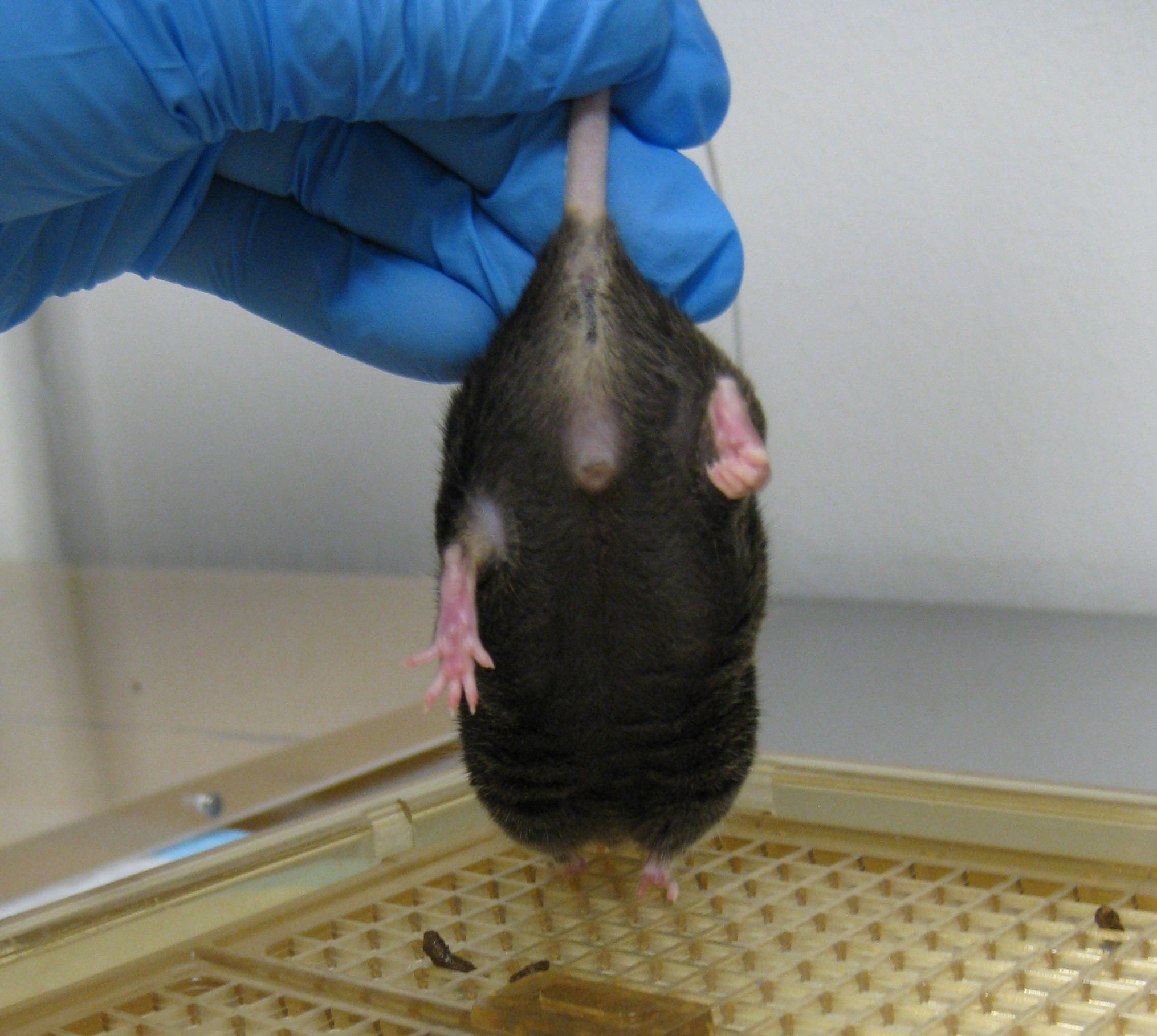
Figure 10. Impaired hindlimb flexion - Forelimb flexion is similar to hindlimb flexion test (Fujimoto et al., 2004). Grasp the mouse by the base of the tail and lift the mouse. Gently and slowly lower the mouse head first towards a flat surface. Uninjured animals will immediately extend their forelimbs towards the mat (Figure 11) (Fujimoto et al., 2004). Use the following parameters to score the left and right forelimbs:
- 4 (no impairment)-Forelimb is extended forward, away from the animal’s body and toes are spread.
- 3-If the forelimb briefly retracts towards the abdomen less than 25% of the time, and toes are spread.
- 2-If the forelimb retracts towards the abdomen more than 25% of the time, but less than 50% of the time, and toes are curled.
- 1-If the forelimb retracts towards the abdomen more than 50% of the time, and toes are curled.
- 0 (severely impaired)-If the forelimb is entirely retracted while touching the abdomen and toes are curled for the entire time while lifted.
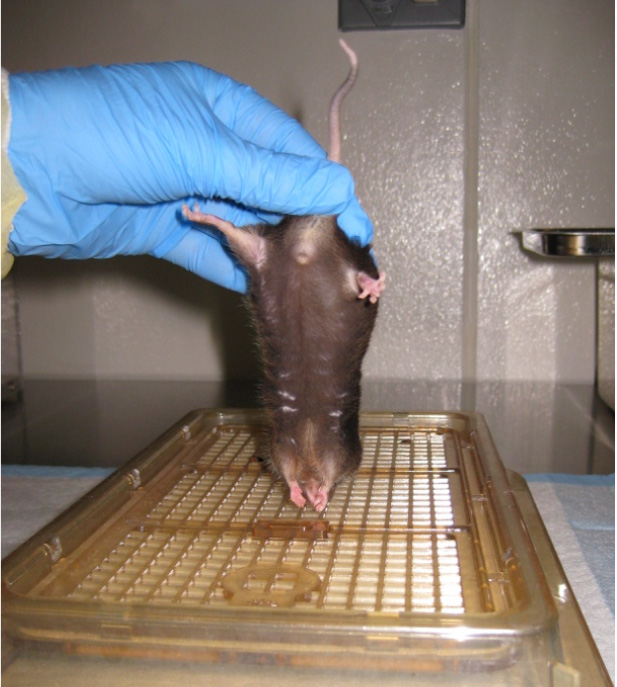
Figure 11. Forelimb flexion
- Lateral pulsion task-The lateral pulsion task tests the animal's ability to coordinate the movements of all four limbs when pressure is applied laterally.
- Place the mouse facing away from the observer. Wipe a pen down with 70% isopropanol and apply gentle, lateral pressure to the mouse's midsection. Uninjured animals will show complete resistance to falling, while injured animals will show little resistance (Figure 12) (Fujimoto et al., 2004). Score the behavior using the following parameters:
- 4 (no impairment)-Complete resistance to falling and no steps taken away from stimulus.
- 3-Moderate resistance, and one step taken with forelimb away from the stimulus.
- 2-Moderate resistance, and more than one step taken away from stimulus.
- 1-Slight resistance, but mouse eventually falls laterally.
- 0 (severely impaired)-No resistance, and mouse falls laterally.
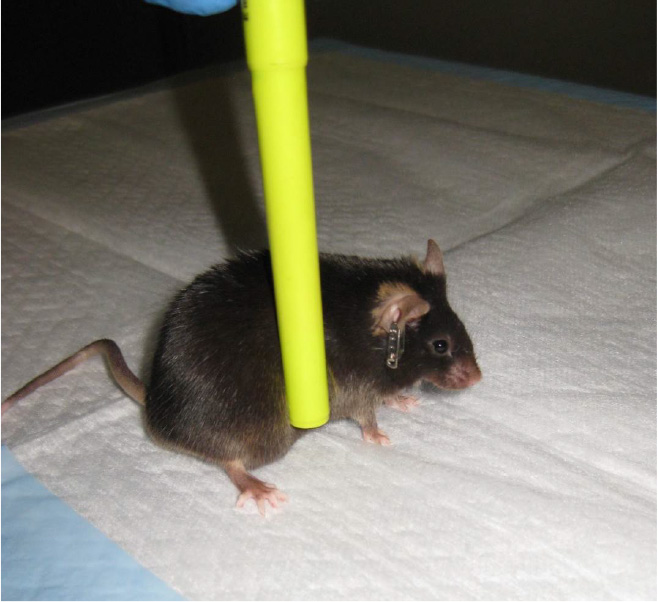
Figure 12. Lateral pulsion
- Place the mouse facing away from the observer. Wipe a pen down with 70% isopropanol and apply gentle, lateral pressure to the mouse's midsection. Uninjured animals will show complete resistance to falling, while injured animals will show little resistance (Figure 12) (Fujimoto et al., 2004). Score the behavior using the following parameters:
- Inclined plane test-The difference between the highest angles an animal can stay on an inclined plane without falling at baseline and after injury typically correlates with the severity of the brain injury (Fujimoto et al., 2004). The angle board device is 50 x 60 cm in size and is covered with a vertically grooved rubber mat (Figure 2). Baseline values should be collected the day before CCI surgery. These values are compared to post-TBI scores.
- Baseline scoring: Set the angle board at 40° and make sure it is secure. Clean any hair on the board using the vacuum.
Note: Do not clean the board with alcohol or any other liquid. Note the room temperature. - Place the mouse in the vertical direction first, then the left, and then the right. A successful completion is determined when the animal is able to stand still in that direction for 5 sec without being held by the tail. Each direction is allotted three attempts (Figure 13).
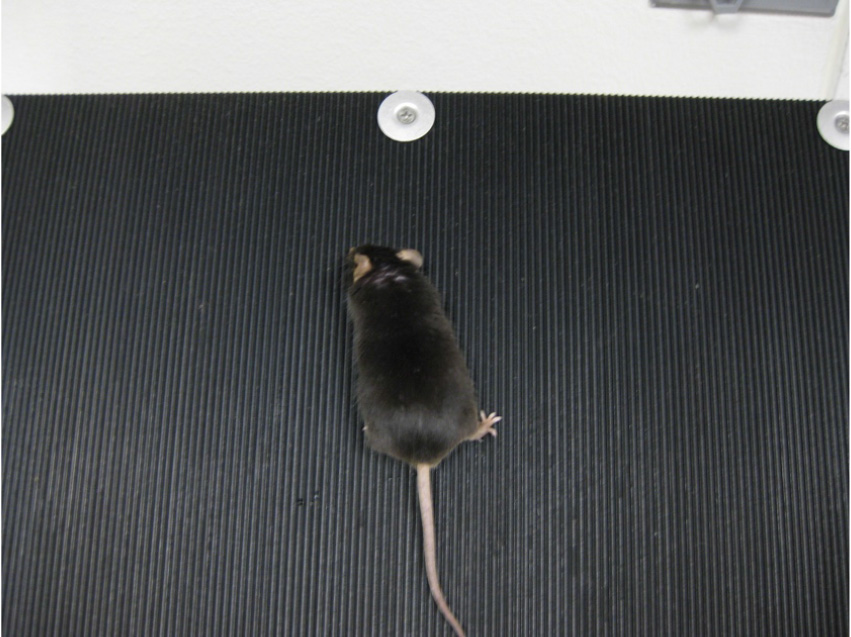
Figure 13. Inclined plane test - If the mouse succeeds in standing on the board, increase the angle by 2.5° and repeat step C2 for each direction. Continue increasing the angle until the mouse can no longer stand on the board in any of the directions. Note the maximum angles.
- Post-TBI scoring: Post-injured animals start testing at 10° below their lowest maximum baseline value. For example, if a mouse fell in the right direction at 47.5°, the left direction at 50°, and the vertical direction at 55°, the starting angle for post-injury testing would be 37.5° (10° less than the right direction maximum angle).
- Scores are assigned for each direction by comparing the post-injury values to the baseline maximum angles. The three scores are then averaged for a final score. Use the following parameters to score the performances:
- 4 (no impairment)-There is no difference or the angle was higher than the baseline maximum angle.
- 3-There is a 2.5° decrease from the baseline maximum angle.
- 2-There is a 5° decrease from the baseline maximum angle.
- 1-There is a 7.5° decrease from the baseline maximum angle.
- 0 (severely impaired)-There is a 10° or more decrease from the baseline maximum angle.
- Baseline scoring: Set the angle board at 40° and make sure it is secure. Clean any hair on the board using the vacuum.
- Hindlimb and forelimb flexion
The composite neuroscore is generated by combining the scores of all seven tests.
Acknowledgments
This study was supported by the Department of Anesthesiology and Pain Medicine of the University of Washington and by NIH SC1GM095426. This protocol was adapted from what was described in Dixon et al., 1991.
References
- Bolkvadze, T. and Pitkanen, A. (2012). Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma 29(5): 789-812.
- Dixon, C. E., Clifton, G. L., Lighthall, J. W., Yaghmai, A. A. and Hayes, R. L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39(3): 253-262.
- Febinger, H. Y., Thomasy, H. E., Pavlova, M. N., Ringgold, K. M., Barf, P. R., George, A. M., Grillo, J. N., Bachstetter, A. D., Garcia, J. A., Cardona, A. E., Opp, M. R. and Gemma, C. (2015). Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J Neuroinflammation 12: 154.
- Fujimoto, S. T., Longhi, L., Saatman, K. E., Conte, V., Stocchetti, N. and McIntosh, T. K. (2004). Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev 28(4): 365-378.
- Smith, D. H., Soares, H. D., Pierce, J. S., Perlman, K. G., Saatman, K. E., Meaney, D. F., Dixon, C. E. and McIntosh, T. K. (1995). A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma 12(2): 169-178.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Febinger, H. Y., Thomasy, H. E. and Gemma, C. (2016). A Controlled Cortical Impact Mouse Model for Mild Traumatic Brain Injury. Bio-protocol 6(16): e1901. DOI: 10.21769/BioProtoc.1901.
Category
Neuroscience > Behavioral neuroscience > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









