- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Macrophage Polarization by Tumor-induced MDSCs Assay
Published: Vol 6, Iss 16, Aug 20, 2016 DOI: 10.21769/BioProtoc.1900 Views: 14268
Reviewed by: Lee-Hwa TaiClara Lubeseder-MartellatoToshitsugu Fujita

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Adoptive Transfer of Monocytes Sorted from Bone Marrow
Damya Laoui [...] Jo A Van Ginderachter
Jan 5, 2019 9077 Views

A 3D Skin Melanoma Spheroid-Based Model to Assess Tumor-Immune Cell Interactions
Marek Wagner and Shigeo Koyasu
Dec 5, 2020 4588 Views

In vivo Imaging of Tumor and Immune Cell Interactions in the Lung
Richard N. Hanna [...] Catherine C. Hedrick
Oct 20, 2016 10737 Views
Abstract
Myeloid derived suppressor cells (MDSCs) are a subset of granulocytes (immature myeloid cells) that exploit a variety of mechanism to modulate the innate and adaptive immune system. MDSCs are present normally in the body, but their numbers increase during inflammation and in cancer, promoting an immunosuppressive microenvironment. In addition to MDSCs, macrophages also play an important role during cancer development. There are two subsets of tumor associated macrophages (TAMs): M1 and M2. M1 are “anti-tumor” macrophages that are activated by interferon gamma (IFN-γ) and/or Lipopolysaccharide (LPS) and secrete high amount of interleukin 12 (IL-12) thereby inducing a Th1 anti-tumor immune response. M2 or “pro-tumorigenic” macrophages are activated by interleukin 4 (IL-4) and interleukin 10 (IL-10) and secrete large amounts of IL-10, which promotes tumor progression (Gabrilovich et al., 2012).
Interaction between MDSCs and macrophages in the tumor microenvironment was shown to enhance immune suppression mediated by these subsets. MDSCs influence TAMs by producing IL-10 that, in turn, induces a down-regulation of IL-12 and polarizes M1 into M2 macrophages. In our study, we use the following protocol to evaluate the ability of tumor induced MDSCs to polarize LPS activated M1 into M2 macrophages (Vences-Catalan et al., 2015). This protocol was adapted from a previous study (Sinha et al., 2007).
Materials and Reagents
- 70 μm cell strainer (Corning, Falcon®, catalog number: 352350 )
- 1 and 10 ml syringe (BD, catalog number: 309659 and 309604 )
- 15 and 50 ml polypropylene conical tubes (Corning, Falcon®, catalog number: 352096 and 352070 )
- 18 gauze needle (BD, catalog number: 305196 )
- 24-well plates (Corning, Falcon®, catalog number: 351147 )
- Mice
Note: We use 6-8 weeks old female Balb/c mice, but any strain of mice can be used as long as both macrophages and MDSCs are from the same genetic background. - Cells
Note: We use 4T1 breast cancer cell line syngeneic to Balb/c; this tumor model is known to induce a strong accumulation of MDSCs in blood, spleen and tumor. - Thioglycolate (BD, catalog number: 211716 )
Note: A 3% solution in water and sterilized in autoclave at 121 °C for 15 min has been used. - PBS (Corning, Cellgro, catalog number: 21-031-CV )
- RPMI 1640 (Corning, Cellgro, catalog number: 10-040-CV )
- DMEM media (Corning, Cellgro, catalog number: 10-017-CV )
- Penicillin-Streptomycin (Pen-Strep) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-22 )
Note: 5 ml of this solution was used in 500 ml of RPMI 1640 media. - Fetal calf serum (FCS) (GE Life Sciences, HyCloneTM, FetalClone®III, catalog number: SH30109.03 )
- ACK buffer (Quality Biological, catalog number: 118-156-101 )
- IL-12p70 and IL-10 ELISA kit (Biolegend, Legend MaxTM, catalog number: 431417 and 433607 )
- CD11b PE (clone: M1/70) (BD, catalog number: 553311 )
- F4/80 APC (clone: BM8) (eBioscience, catalog number: 17-4801-82 )
- Anti Ly6G and Ly6c (Gr1) APC (clone: RB6-8C5) (BD, catalog number: 553129 )
- LPS 1 mg/ml (Sigma-Aldrich, catalog number: L3012-5MG )
Note: If using a different tumor model where MDSCs in blood or spleen represents a small percentage of total Peripheral Blood Mononuclear Cells or splenocytes, respectively, purify MDSCs with myeloid-derived suppressor cell isolation kit and follow instructions according to manufacturers protocol (Miltenyi Biotec, catalog number: 130-094-538 ).
Equipment
- Centrifuge (Eppendorf, model: 5810 R )
Procedure
Note: Isolation of peritoneal macrophages as well as MDSCs should be performed under sterile conditions.
- Macrophage isolation
- Inject intraperitoneally (i.p.) 3-4 naïve Balb/c mice with 1 ml of sterile 3% thioglycolate 4-5 days before macrophage isolation.
- 4-5 days later, euthanize mice by cervical dislocation (AVMA Guidelines for the Euthanasia of Animals: 2013 Edition). Carefully make a small incision on the skin to expose the peritoneal wall, avoid cutting the peritoneal wall to prevent leakage of the PBS. Harvest peritoneal cells by injection of 5-10 ml of sterile cold PBS into the peritoneal cavity; give a small massage to shed the peritoneal cells (use 18 gauze needle for the extraction, wash several times with PBS). Collect PBS each time you wash the peritoneum (Lu, 2013).
- Spin the cells at 300 x g for 5 min, count the cells and resuspend in RPMI or DMEM media containing 10% FCS and 1% Pen-Strep. If red blood cells are present, lyse with ACK buffer. Briefly, add 3-5 ml of ACK lysis buffer and incubate for 5 min. Quench the reaction by adding two times the volume of PBS. Spin for 5 min, 300 x g and repeat wash one more time.
- Plate cells at 7.5 x 105 cells/well/500 μl of RPMI or DMEM/10% FCS and 1% Pen-Strep in 24-well plates, and incubate at 37 °C in 5% CO2 for 3 h.
- Remove non-adherent cells, and wash the attached cells once with RPMI or DMEM media.
- Save an aliquot (to detach the cells add 2 mM EDTA in PBS for approximately 10-15 min, collect the cells and spin down for 5 min at 300 x g), resuspend in PBS and evaluate the purity of your macrophage population by staining with CD11b and F4/80 antibodies (1 μg of antibody per million of cells) by flow cytometry, as illustrated in Figure 1.
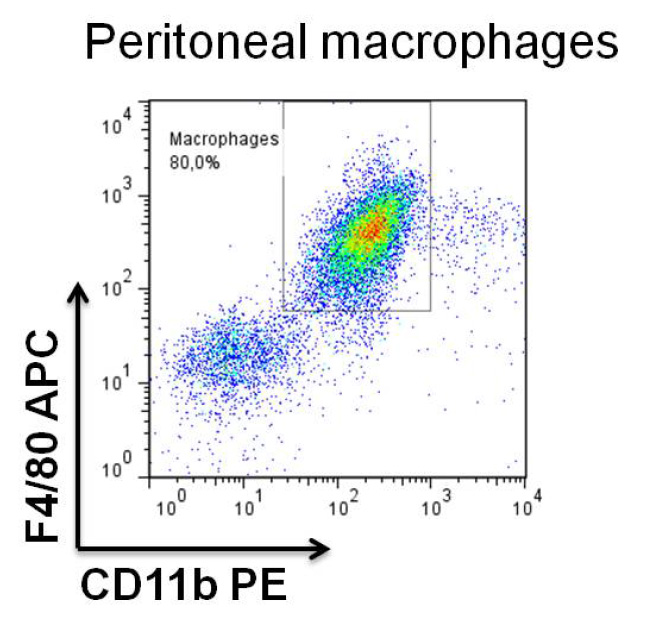
Figure 1. Analysis of peritoneal macrophages
- Tumor derived blood MDSC isolation
- Inject 1 x 104-1 x 105 4T1 cells subcutaneously into the mammary fat pad of female Balb/c mice (Reuter, 2011). On day 21-28 almost 90% of white blood cells are MDSCs (doubled positive CD11b+Gr1+), as seen in Figure 2. Determine the percentage of Gr1 positive MDSCs by staining with anti Gr-1- APC and CD11b- PE antibodies and analyze by flow cytometry (1 μg of antibody per million of cells).
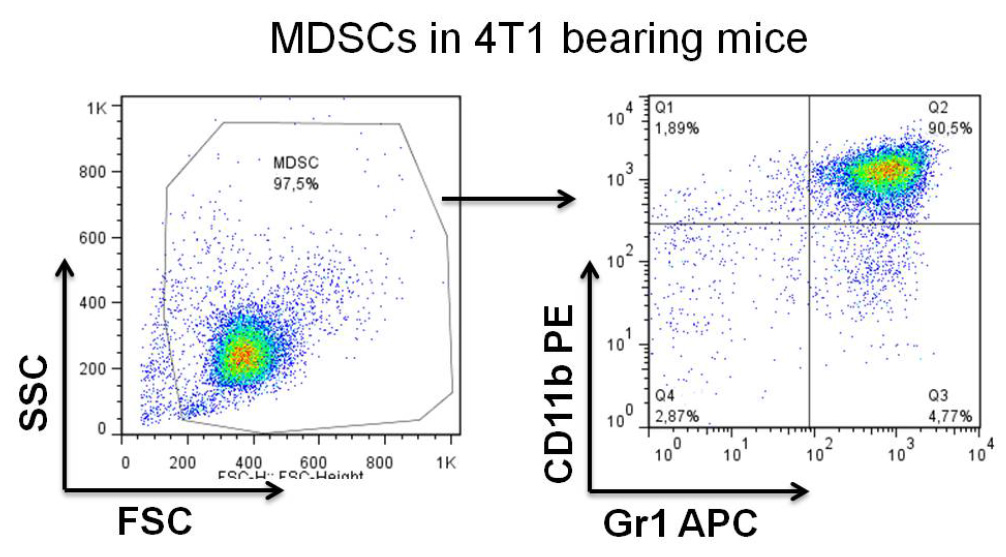
Figure 2. Analysis of MDSCs in the blood of day 28 of 4T1-tumor bearing mice - On day 21-28 after tumor implantation, collect 100-500 μl blood by cardiac puncture from tumor bearing animals and lyse red blood cells with ACK buffer for 3-5 min at RT (5 ml of ACK for 500 μl of blood). Wash cells twice with PBS by spinning two times at 300 x g for 5 min at RT, count cells and resuspend in complete media.
Optional: If MDSCs represent a minor percentage of total white blood cells, purify MDSCs with myeloid-derived suppressor cell isolation kit. - Co-culture MDSCs (1.5 x 106 MDSCs/well/500 μl of RPMI or DMEM/10% FCS and 1% Pen-Strep media) with 7.5 x 105 macrophages by mixing them together in the same well (or at with different ratios MDSCs:Macrophages, 2:1, 1:1, etc.) in triplicates, add 100 ng/ml of LPS and incubate for 16-18 h at 37 °C, 5% CO2. Include wells that contain only macrophages and only MDSCs as controls (as represented in Figure 3) as well as in the presence and absence of LPS, as illustrated in Figure 4.
- After 16-18 h, collect culture supernatants and measure IL-12 and IL-10 secretion by ELISA (Remove cell debris by spinning 3 min 800 x g). Optional is detection of IFN-γ secretion according to the manufacturer’s protocol.
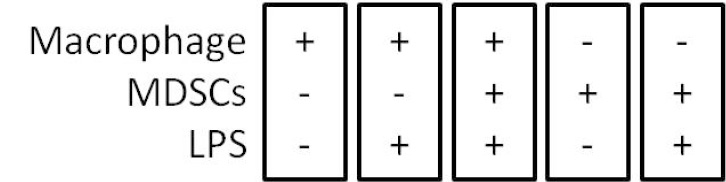
Figure 3. Representative scheme of the co-culture of macrophages with MDSC’s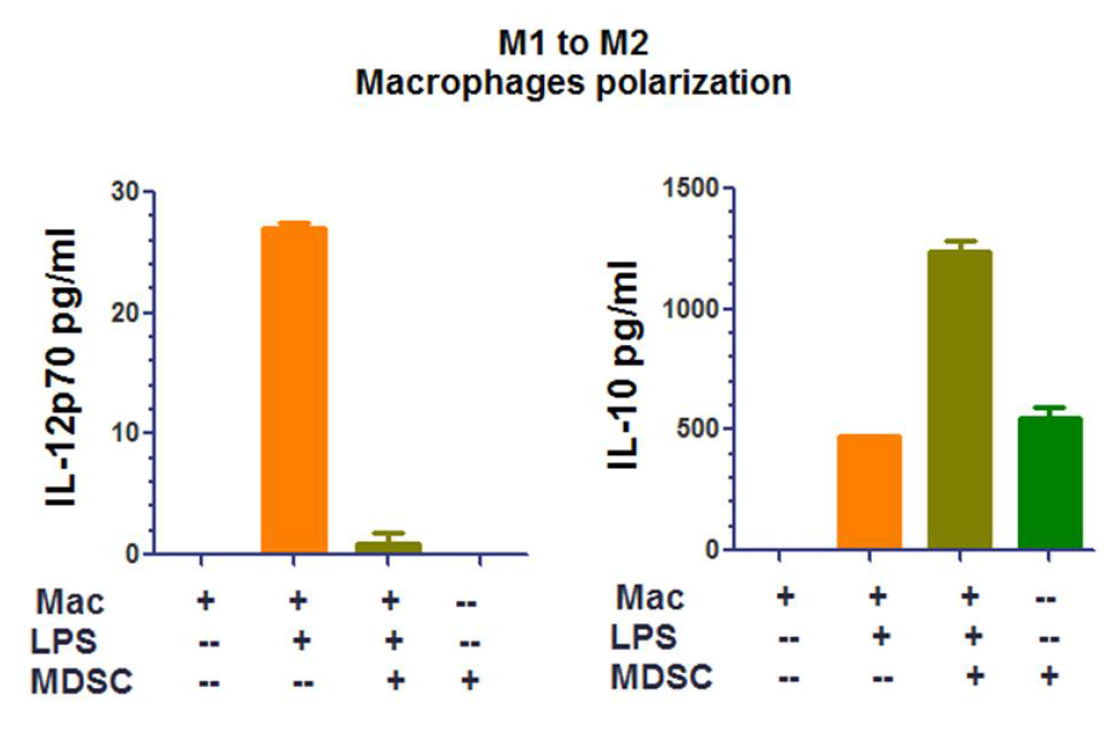
Figure 4. Representative analysis of a macrophage polarization by MDSCs assay
- Inject 1 x 104-1 x 105 4T1 cells subcutaneously into the mammary fat pad of female Balb/c mice (Reuter, 2011). On day 21-28 almost 90% of white blood cells are MDSCs (doubled positive CD11b+Gr1+), as seen in Figure 2. Determine the percentage of Gr1 positive MDSCs by staining with anti Gr-1- APC and CD11b- PE antibodies and analyze by flow cytometry (1 μg of antibody per million of cells).
Acknowledgments
This work was supported by the Translational Cancer Award from Stanford Cancer Institute and the Breast Cancer Research program from the Department of Defense grant W81XWH-14-1-0397. This protocol was adapted from a previous study (Sinha et al., 2007).
References
- Gabrilovich, D. I., Ostrand, R. S. and Bronte, V. (2012). Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12(4): 253-268.
- Lu, M. and Varley, A. W. (2013). Harvest and culture of mouse peritoneal macrophages. Bio-protocol 3(22): e976.
- Reuter, J. (2011). Subcutaneous injection of tumor cells. Bio-protocol Bio101: e166.
- Sinha, P., Clements, V. K., Bunt, S. K., Albelda, S. M. and Ostrand-Rosenberg, S. (2007). Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. Journal of Immunology 179(2): 977-983.
- Vences-Catalan, F., Rajapaksa, R., Srivastava, M. K., Marabelle, A., Kuo, C. C., Levy, R. and Levy, S. (2015). Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res 75(21): 4517-4526.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Vences-Catalán, F., Srivastava, M. K. and Levy, S. (2016). Macrophage Polarization by Tumor-induced MDSCs Assay. Bio-protocol 6(16): e1900. DOI: 10.21769/BioProtoc.1900.
Category
Immunology > Immune cell function > Myeloid derived suppressor cells
Cancer Biology > Tumor immunology > Tumor microenvironment > Immunosuppression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











