- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adoptive Transfer of Tumor Expanded Regulatory T Cells (Tregs)
Published: Vol 6, Iss 16, Aug 20, 2016 DOI: 10.21769/BioProtoc.1899 Views: 11321
Reviewed by: Lee-Hwa TaiClara Lubeseder-MartellatoToshitsugu Fujita

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A 3D Skin Melanoma Spheroid-Based Model to Assess Tumor-Immune Cell Interactions
Marek Wagner and Shigeo Koyasu
Dec 5, 2020 4588 Views

Expansion and Polarization of Human γδT17 Cells in vitro from Peripheral Blood Mononuclear Cells
Xu Chen [...] Jun Yan
Jan 5, 2024 1951 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2620 Views
Abstract
Regulatory T cells (Tregs), a subset of CD4+CD25+ T cells, infiltrate tumors and suppress antitumor activity of effector T and NK cells. Depletion of Tregs by anti CD25+ antibodies has been shown to reduce tumor growth and metastasis (Olkhanud et al., 2009). Conversely, adoptive transfer of Tregs induced immune suppression and promoted tumor growth (Smyth et al., 2006; Janakiram et al., 2015). We have adoptively transferred Tregs to evaluate their immunosuppressive function in vivo. Our study (Vences-Catalan et al., 2015) compared the immunosuppressive efficacy of Tregs derived from tumor-bearing wild type to those of CD81KO mice. The following protocol could be adapted to any other source of Tregs.
Lymph node or splenic tumor-induced Tregs are isolated and purified by a two-step procedure using CD4+CD25+ regulatory T cell isolation kit from MACS Miltenyi Biotec. First, CD4+ T cells are enriched by negative selection, followed by positive selection of CD25+ T cells. Tumor-induced purified Tregs (CD3+CD4+CD25+FoxP3+) are then co-injected subcutaneously together with tumor cells into naïve mice (Winn assay) (Winn, 1960). Tregs could also be injected intravenously once or several times, according to the research needs. The effect of the adoptively transferred Tregs on tumor growth is then measured by caliper or by in vivo imaging techniques.
Materials and Reagents
- 70 μm cell strainer (Corning, Falcon®, catalog number: 352350 )
- 1 ml syringe (BD, catalog number: 309659 )
- 30 G x 1/2 needle (BD, catalog number: 305106 )
- LD columns (Miltenyi Biotec, catalog number: 130-042-901 )
- MS columns (Miltenyi Biotec, catalog number: 130-042-201 )
- 15 and 50 ml polypropylene conical tubes (Corning, Falcon®, catalog number: 352096 and 352070 )
- Mice
Note: We use 6-8 weeks old female Balb/c or C57BL6 mice, but the protocol is applicable to any mouse strain as long as both donor and recipient mice are from the same genetic background. - Cells
Note: We use breast cancer cell lines 4T1 or E0771 syngeneic to Balb/c or C57Bl6 respectively. - Fetal calf serum (FCS) (GE life sciences, HyCloneTM, FetalClone®III, catalog numer: SH30109.03 )
- Penicillin-Streptomycin (Pen-Strep) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-22 )
Note: 5 ml of this solution was used in 500 ml of RPMI 1640 media. - PBS (Corning, Cellgro, catalog number: 21-031-CV )
- ACK buffer (Quality Biological, catalog number: 118-156-101 )
- CD4+CD25+ Regulatory T cell Isolation kit (Miltenyi Biotec, catalog number: 130-091-041 )
Components of the kit:- 1 ml CD4+CD25+ Regulatory T cell Biotin-antibody cocktail
- 2 ml Anti-biotin Microbeads
- 1 ml CD25-PE
- 1 ml Anti-PE Microbeads
- RPMI 1640 (Corning, Cellgro, catalog number: 10-040-CV )
- Bovine serum albumin (BSA) (Sigma Aldrich, catalog number: A9647-500G )
Note: A solution of 0.5% in PBS was used. - EDTA (Sigma Aldrich, catalog number: E-5134 )
Note: A solution of 2 mM in PBS was used. - Optional: CD3 FITC (clone:145-2C11) (BD Pharmingen)
- Optional: CD25 PE (clone:PC61.5) (BD Pharmingen)
- Optional: CD4 PerCP (clone:H129.19/RM4-5) (BD Pharmingen)
- Optional: FoxP3 APC (clone:FJK16s) (BD Pharmingen)
- MACS Buffer (see Recipes)
Equipment
- MidiMACS separator (Miltenyi Biotec, catalog number: 130-042-302 )
- MiniMACS separator (Miltenyi Biotec, catalog number: 130-042-102 )
- Centrifuge (Eppendorf, model: 5810 R )
Procedure
- Induction of regulatory T cells
4T1 or E0771 cells were grown in RPM media, supplemented with 10% FCS and 1% Pen-Strep. Inject subcutaneously 1-5 x 104 4T1 or 0.5-1 x 106 E0771 cells resuspended in PBS (90% or greater viability) into either Balb/c or C57BL6 mice, respectively (an average of 3-5 mice are needed to obtain around 1-5 x 106 Tregs, if more Tregs are needed inject more mice). - Sample preparation for Treg purification
After 12-16 days, euthanize the mice by cervical dislocation (AVMA Guidelines for the Euthanasia of Animals: 2013 Edition) and remove lymph nodes (inguinal and axillary) (Mathieu et al., 2012) and/or spleens. Prepare single-cell suspension by mechanical disruption of the tumor (make sure to work with single cell suspension, by passing through a 70 μm cell strainer).
Optional: If red blood cells are present, lyse with ACK buffer, although it is not necessary as the kit contains anti Ter-119 antibody to positively deplete red blood cells.
Note: Work fast, keep cells cold and use pre-cooled solutions. This will prevent capping of antibodies on the cell surface and will reduce non-specific cell labeling. - Magnetic labeling of non-CD4+ cells and fluorescent labeling of CD25+ cells
We follow exactly the protocol provided by the manufacturer to purify CD4+CD25+ Regulatory T cells. Volumes for magnetic labeling given below are for starting cell number of 107 leukocytes. When working with fewer than 107 leukocytes use the same volumes as indicated. When working with higher cell numbers, scale up all reagent volumes and total volumes accordingly.- Determine the number of leukocytes.
- Centrifuge cells at 300 x g for 10 min at room temperature (RT). Aspirate supernatant completely.
- Resuspend cell pellet in 40 μl of MACS buffer per 107 total cells.
- Add 10 μl of Biotin-Antibody cocktail per 107 total cells (this cocktail contains anti-mouse antibodies against: CD8a, CD11b, CD45R, CD49b and Ter-119).
- Mix well by pipetting up and down, and refrigerate for 10 min (4-8 °C).
- Add 30 μl of MACS buffer, 20 μl of anti-biotin Microbeads and 10 μl of CD25-PE antibody per 107 total cells.
- Mix well and refrigerate for an additional 15 min in the dark (4-8 °C).
- Wash cells by adding 1-2 ml of buffer per 107 total cells and centrifuge at 300 x g for 10 min RT. Aspirate supernatant completely.
- Resuspend cell pellet in 500 μl of MACS buffer (up to 1.25 x 108 cells can be suspended in this volume according to the manufacturer).
- Proceed to magnetic separation.

Figure 1. MACS midi system for cell purification
- Depletion with LD column
- Place LD column in the magnetic field of a midi MACS separator (Figure 1).
- Prepare column by rinsing with 2 ml of MACS buffer.
- Apply cell suspension to the column.
- Collect unlabeled cells which pass through and wash column 2 times by pipetting 1 ml of MACS buffer. Perform washing steps by adding MACS buffer successively once the column reservoir is empty. Collect total effluent. This is the unlabeled CD4+ T cell fraction.
- Proceed for the enrichment of CD4+CD25+ T cells.
- Magnetic labeling of CD25+ cells
Volumes for magnetic labeling given below are for up to 107 initial starting cell number. For larger initial cell numbers, scale up accordingly.- Centrifuge isolated CD4+ T cells at 300 x g for 10 min. Aspirate supernatant completely.
- Resuspend cell pellet in 90 μl of MACS buffer.
- Add 10 μl of anti-PE Microbeads.
- Mix well and refrigerate for 15 min in the dark (4-8 °C).
- Wash cells by adding 1-2 ml of MACS buffer and centrifuge at 300 x g for 10 min. Aspirate supernatant completely.
- Resuspend up to 108 cells in 500 μl of MACS buffer.
- Proceed to magnetic separation.
- Magnetic separation: Positive selection of CD4+CD25+ regulatory T cells
- Place MS column in the magnetic field of a suitable MACS separator.
- Prepare column by rinsing with 500 μl of MACS buffer.
- Apply cell suspension onto the column.
- Let cells pass through and wash column 3 times with 500 μl of MACS buffer. Perform washing steps by adding MACS buffer three times, once the column reservoir is empty.
- Remove column from the separator and place it on a suitable collection tube.
- Pipette 1 ml of MACS buffer on to the column. Immediately flush out the magnetically labeled cells (CD4+CD25+) by firmly pushing the plunger into the column.
- Optional: To achieve highest purities, repeat magnetic separation with a consecutive column run.
- Co-injection of Tregs with 4T1 or E0771 tumor cells
- Determine the cell number of Tregs and tumor cells needed for injection.
- Inject approximately 100-200 μl of PBS containing a mixture purified Tregs with tumor cells subcutaneously close to the mammary fat pad.
- Follow tumor growth by caliper measurements.
Representative data
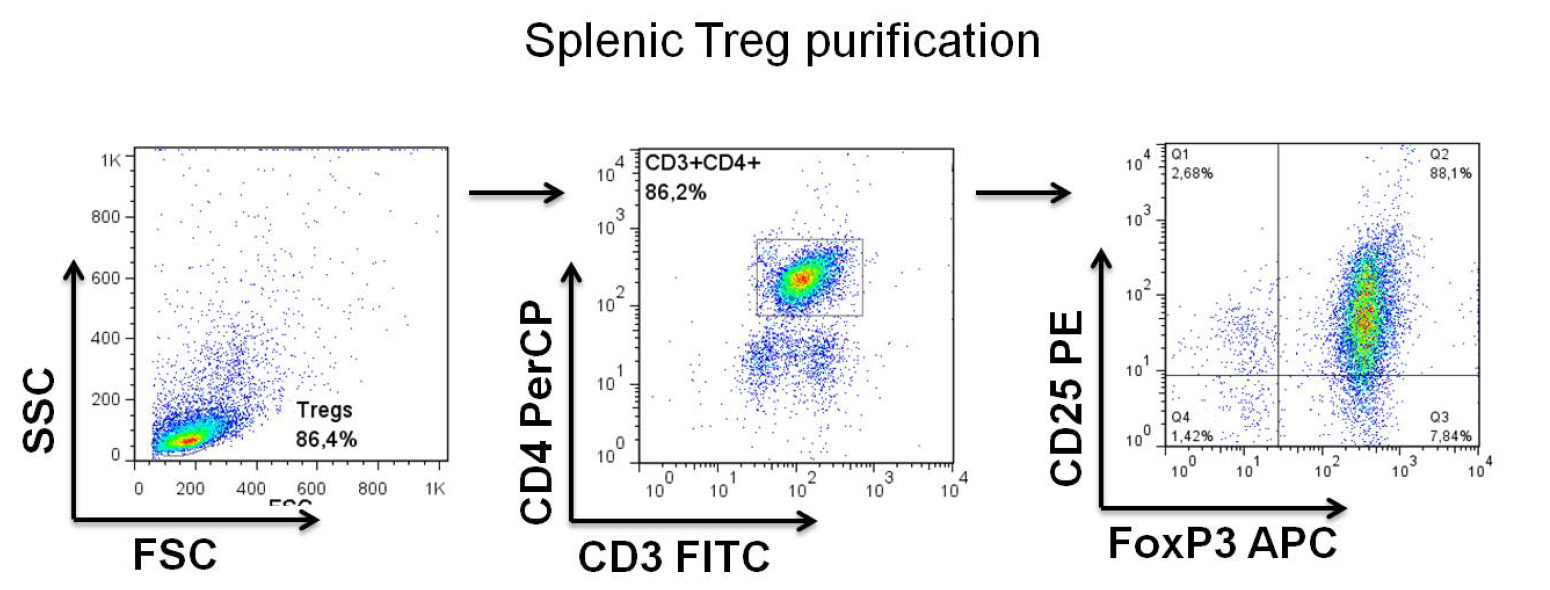
Figure 2. Representative Treg purification
Notes
- Tregs purity varies from 85-95% (See Figure 2).
- On our experiments we transferred 5 x 104 4T1 cells together with 1 x 106 Tregs or 0.5 x 106 E0771 cells with 1 x 106 Tregs.
Recipes
- MACS Buffer
PBS supplemented with 0.5% BSA and 2 mM EDTA
Keep buffer cold (4-8 °C)
Acknowledgments
This work was supported by the Translational Cancer Award from Stanford Cancer Institute and the Breast Cancer Research program from the Department of Defense grant W81XWH-14-1-0397. Adapted from (Winn, 1960).
References
- Janakiram, N. B., Mohammed, A., Bryant, T., Brewer, M., Biddick, L., Lightfoot, S., Lang, M. L. and Rao, C. V. (2015). Adoptive transfer of regulatory T cells promotes intestinal tumorigenesis and is associated with decreased NK cells and IL-22 binding protein. Mol Carcinog 54(10): 986-998.
- Mathieu, M. and Labrecque, N. (2012). Murine superficial lymph node surgery. J Vis Exp (63): e3444.
- Olkhanud, P. B., Baatar, D., Bodogai, M., Hakim, F., Gress, R., Anderson, R. L., Deng, J., Xu, M., Briest, S. and Biragyn, A. (2009). Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res 69(14): 5996-6004.
- Smyth, M. J., Teng, M. W., Swann, J., Kyparissoudis, K., Godfrey, D. I. and Hayakawa, Y. (2006). CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 176(3): 1582-1587.
- Vences-Catalan, F., Rajapaksa, R., Srivastava, M. K., Marabelle, A., Kuo, C. C., Levy, R. and Levy, S. (2015). Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res 75(21): 4517-4526.
- Winn, H. J. (1960). The immune response and the homograft reaction. Natl Cancer Inst Monogr 2: 113-138.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Vences-Catalán, F. and Levy, S. (2016). Adoptive Transfer of Tumor Expanded Regulatory T Cells (Tregs). Bio-protocol 6(16): e1899. DOI: 10.21769/BioProtoc.1899.
Category
Immunology > Immune cell isolation > Lymphocyte
Cancer Biology > Tumor immunology > Tumor microenvironment
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










