- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Purification of Flagellin from Acidovorax avenae and Analysis of Plant Immune Responses Induced by the Purified Flagellin
Published: Vol 6, Iss 16, Aug 20, 2016 DOI: 10.21769/BioProtoc.1898 Views: 9201
Reviewed by: Zhaohui LiuChong HeArsalan Daudi

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Biofilm Assays on Fibrinogen-coated Silicone Catheters and 96-well Polystyrene Plates
Cristina Colomer-Winter [...] Ana L. Flores-Mireles
Mar 20, 2019 7645 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2775 Views

Immunofluorescence for Detection of TOR Kinase Activity In Situ in Photosynthetic Organisms
Ana P. Lando [...] Giselle M. A. Martínez-Noël
Dec 20, 2024 1824 Views
Abstract
Plants sense potential pathogens by recognizing conserved pathogen-associated molecular patterns (PAMPs) that cause PAMP-triggered immunity (PTI) including the generation of reactive oxygen species, callose deposition, and expression of several PTI-related genes. Acidovorax avenae is a Gam-negative bacterium that causes a seedling disease characterized by the deposition of brown stripes on the sheaths of infected plants. We previously reported that flagellin isolated from the rice avirulent A. avenae N1141 strain induces PTI, while flagellin isolated from the rice virulent A. avenae K1 strain does not induce PTI. To examine the molecular mechanism of specific PTI induction by N1141 flagellin, highly purified flagellin from N1141 or K1 strains is required. Here, we describe a high quality purification method for the A. avenae flagellins and for using it in PTI induction study.
Keywords: FlagellinMaterials and Reagents
- 0.22 µm sterilization filter (Merck Millipore, catalog number: SLGS033SB )
- Nitrocellulose membrane (GE Healthcare, code number: 10401196 )
- Collodion-coated grid (Ted Pella, Inc., catalog number: 12575-CU )
- Cultured rice cells of line OC (Oryza sativa C5928; obtained from RIKEN BioResource Center)
- Acidovorax avenae N1141 (MAFF 301141) and K1 (MAFF301755) (National Institute of Agrobaiological Sciences genebank, catalog number: MAFF 301141 ; MAFF301755 )
- Skim milk powder (Wako, catalog number: 190-12865 )
- Sodium hydrogen L(+)-glutamate monohydrate (Wako, catalog number: 198-02035 )
- Luria-Bertani (LB) liquid medium (MO BIO laboratories, catalog number: 12107-05 )
- 2-amino-2-hydroxymethyl-1,3-propanediol (Tris) (Wako, catalog number: 204-07885 )
- NaCl (Nacalai Tesque, catalog number: 31320-05 )
- KCl (Nacalai Tesque, catalog number: 28514-75 )
- 1% (w/v) phosphotungstic acid (pH 6.9) (Sigma-Aldrich, catalog number: 79690 )
- Sodium dodecyl sulfate (SDS) (Wako, catalog number: 191-07145 )
- Molecular-weight marker low (APRO, catalog number: SP-0110 )
- Anti-flagellin rabbit antibody (Eurofins Genomics)
Note: Flagellin purified from A. avenae N1141 strain as antigen was injected in rabbit. Anti-flagellin rabbit antibody was purified from rabbit antiserum (anti-flagellin) using flagellin purified from A. avenae N1141 strain. - Goat HRP conjugated anti-rabbit IgG antibody (H + L chain) [Medical & Biological Laboratories (MBL), catalog number: 458 ]
- ECL plus Western blotting detection reagents (GE Healthcare, code number: RPN2132 )
- Hybri-Bag (Cosmo Bio, catalog number: S-1001 )
- Potassium ferricyanide (Wako, catalog number: 169-03721 )
- KNO3 (Nacalai Tesque, catalog number: 28704-85 )
- (NH4)2SO4 (Wako, catalog number: 019-03435 )
- MgSO4·7H2O (Nacalai Tesque, catalog number: 21003-75 )
- CaCl2·2H2O (Nacalai Tesque, catalog number: 06731-05 )
- NaH2PO4·2H2O (Wako, catalog number: 192-02815 )
- MnSO4·5H2O (Nacalai Tesque, catalog number: 21229-35 )
- ZnSO4·7H2O (Nacalai Tesque, catalog number: 37011-75 )
- CuSO4·5H2O (Nacalai Tesque, catalog number: 09605-04 )
- H3BO3 (Wako, catalog number: 021-02195 )
- Na2MoO4·2H2O (Nacalai Tesque, catalog number: 31621-52 )
- EDTA·2Na (DOJINDO, catalog number: 345-01865 )
- FeSO4·7H2O (Wako, catalog number: 098-01085 )
- MS vitamin powder 1,000x (Sigma-Aldrich, catalog number: M7150-100ML )
- 2, 4-dichlorophenoxyacetic acid (Wako, catalog number: 040-18532 )
- Sucrose (Wako, catalog number: 196-00015 )
- 3-mercapto-1,2-propanediol (Wako, catalog number: 131-16451 )
- Glycerol (Nacalai Tesque, catalog number: 17018-25 )
- Bromophenol blue (BPB) (Wako, catalog number: 021-02911 )
- Glycine (Wako, catalog number: 077-00735 )
- Coomassie brilliant blue R-250 (CBB) (Wako, catalog number: 031-17922 )
- Methanol (Wako, catalog number: 137-01823 )
- Acetic acid (Wako, catalog number: 017-00251 )
- Polyoxyethylene sorbitan monolaurate (Tween 20) (Wako, catalog number: 167-11515 )
- KH2PO4 (Wako, catalog number: 169-04245 )
- Skimmed milk solution (see Recipes)
- Acidovorax avenae N1141 and K1 strain skimmed milk stock solution (see Recipes)
- 25 mM TBS buffer (pH 7.4) (see Recipes)
- 20x R2 Major solution (see Recipes)
- 1,000x R2 Minor solution (see Recipes)
- 500x Fe liquid solution (see Recipes)
- 1,000x MS vitamin solution (see Recipes)
- 2, 4-dichlorophenoxyacetic acid solution (see Recipes)
- R2S medium (pH 5.6) (see Recipes)
- 2x sample buffer (pH 6.8) (see Recipes)
- Electrophoresis buffer (see Recipes)
- CBB staining solution (see Recipes)
- Destaining solution (see Recipes)
- Transfer buffer (see Recipes)
- Tris buffered saline with Tween 20 (TBST) buffer (see Recipes)
- Blocking buffer (see Recipes)
- 50 mM potassium phosphate buffer (pH 7.9) (see Recipes)
- Luminol solution (see Recipes)
- Potassium ferricyanide solution (see Recipes)
Equipment
- Rotary shaker (TAITEC, model: NR-20 )
- High-speed refrigerated micro centrifuge (TOMY, model: MX-300 )
- High-speed refrigerated centrifuge (Hitachi koki, model: CR20G )
- Ultracentrifuge (Hitachi koki, model: CP70MX )
- Fiber blender (Panasonic, model: MX-X58-SW )
- Incubator (TAITEC, model: BR-42FL· MR )
- Transmission electron microscope (Hitachi, model: H-7100 )
- Plant growth chamber (NK system, model: LH-411SP )
- Electrophoresis tank (ATTO, model: AE-6530M )
- Semi-dry blotter (Bio-Rad, catalog number: 1703940JA )
- Luminescent image analyzer (GE Healthcare, model: ImageQuant LAS 4000 )
- Lumi-counter (ATTO, model: AB-2350 )
Procedure
- Purification of flagellar filaments from Acidovorax avenae
- For pre-culture of Acidovorax avenae, add 2 µl of A. avenae skimmed milk stock solution to 2 ml of LB liquid medium, and shake (200 rpm) for overnight at 30 °C.
- Add 100 µl of pre-cultured suspension to one liter fresh LB medium, and shake (200 rpm) for 24 h at 30 °C.
- Harvest A. avenae cells by centrifugation at 6,000 x g for 20 min at room temperature.
- Add 300 ml of 25 mM TBS buffer to the pellet and re-suspend. After centrifugation at 6,000 x g for 20 min at room temperature, discard the supernatant.
- Re-suspend the pellet with 90 ml of TBS buffer, and transfer to fiber blender.
- To take off flagellum from bacterial cells, shear with fiber blender for 1 min at 4 °C, and incubate for 5 min on ice. Repeat this step 7 times.
- To remove intact bacterial cells, centrifuge the bacterial suspension at 6,000 x g for 30 min at 4 °C and save the supernatant.
- To remove bacterial cellular debris, centrifuge the supernatant at 16,000 x g for 60 min at 4 °C and save the supernatant.
- Ultracentrifuge the supernatant at 200,000 x g for 60 min at 4 °C and discard the supernatant.
- Add 1.5 ml of ice-cold distilled water to the pellet and re-suspend.
- Centrifuge the re-suspended pellet at 20,000 x g for 20 min at 4 °C, and discard the supernatant. The pellet contains the monomer and polymer flagellins.
- Re-suspend the pellet with 0.5 ml of ice-cold distilled water and store at -80 °C.
- For pre-culture of Acidovorax avenae, add 2 µl of A. avenae skimmed milk stock solution to 2 ml of LB liquid medium, and shake (200 rpm) for overnight at 30 °C.
- Observation of purified flagellar filaments using transmission electron microscopy
- 50 µl droplet of the purified flagellar filaments (pellet of 20,000 x g) are used.
- Absorb the purified flagellum onto collodion-coated grids, which are supported with carbon and rendered the carbon surface hydrophilic, for 1 min.
- Stain the grids with 1% (w/v) phosphotungstic acid (pH 6.9) for 1 min and wash with two drops of distilled water.
- Images are taken as digitized pictures with transmission electron microscope operated at 80 kV.
- 50 µl droplet of the purified flagellar filaments (pellet of 20,000 x g) are used.
- Purity check of flagellin in the purified flagellar filaments by Western blotting
- Flagellar filaments of A. avenae consists of only flagellin protein. Purity of flagellin in the flagellar filaments is analyzed by SDS-PAGE and Western blotting.
- Add half volume of 2x sample buffer to 100 ng of purified flagellum suspension.
- Heat the sample at 95 °C for 5 min, and cool on ice.
- Load 20 ng of sample and 2 µl of Prestained XL-Ladder Low marker of low range onto SDS-PAGE gel.
- Electrophoresis at constant 100 Volts until the dye reaches the bottom of the gel.
- Electrotransfer to nitrocellulose membrane using transfer buffer (constant 10 Volts for 3 h).
- Incubate membrane in 25 ml of blocking buffer for 1 h at room temperature.
- Wash three times for 5 min each with 25 ml of TBST.
- Incubate membrane with primary Anti-flagellin rabbit antibody (1:2,500) in 10 ml of TBST for 1 h at room temperature.
- Wash five times for 5 min each with 25 ml of TBST.
- Incubate membrane with secondary goat HRP conjugated anti-rabbit IgG antibody (1:2,000) in 10 ml of TBST for 30 min room temperature.
- Wash three times for 5 min each with 25 ml of TBST.
- Incubate membrane with 1 ml of ECL plus Western blotting detection reagent for 3 min at room temperature, and then wrap in Hybri-Bag.
- Measure the intensity of each band with LAS-4000.
- Flagellar filaments of A. avenae consists of only flagellin protein. Purity of flagellin in the flagellar filaments is analyzed by SDS-PAGE and Western blotting.
- H2O2 detection and quantification
- Suspension cultures of rice cells (line OC) were grown at 30 °C under white fluorescent light irradiation in a plant growth chamber. The cells were diluted in fresh medium every week, and experiment for H2O2 detection was performed 4 days after transfer.
- Place the cultured rice cells onto filter paper to remove the culture medium. Add 10 mg culture cells into 0.5 ml of fresh medium, and culture the cells in a plant growth chamber for 2 h at 30 °C.
- Add the flagellin (100 nM) to the cultured cells, and incubate in a plant growth chamber at 30 °C. Less than 100 µl of the flagellin suspension is desirable for addition to a culture medium.
- After incubation, add 10 µl of culture medium to chemiluminescence reaction buffer containing 160 µl of 50 mM potassium phosphate buffer (pH 7.9), 10 µl of 1.1 mM luminol and 20 µl of 14 mM potassium ferricyanide. Quantify H2O2 using a lumi-counter.
- Suspension cultures of rice cells (line OC) were grown at 30 °C under white fluorescent light irradiation in a plant growth chamber. The cells were diluted in fresh medium every week, and experiment for H2O2 detection was performed 4 days after transfer.
Representative data
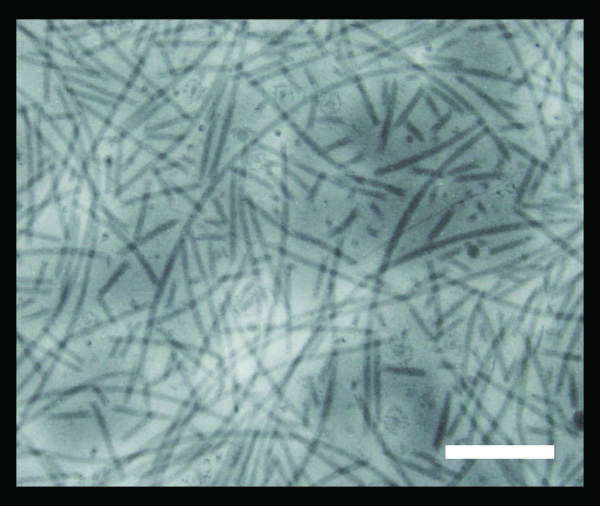
Figure 1. Transmission electron micrograph of flagellar filaments purified from A. avenae K1 strain. The purified sample contains the flagellar filaments of various lengths which are formed by mechanical shearing. Scale bar shown at the bottom right corner is 250 nm.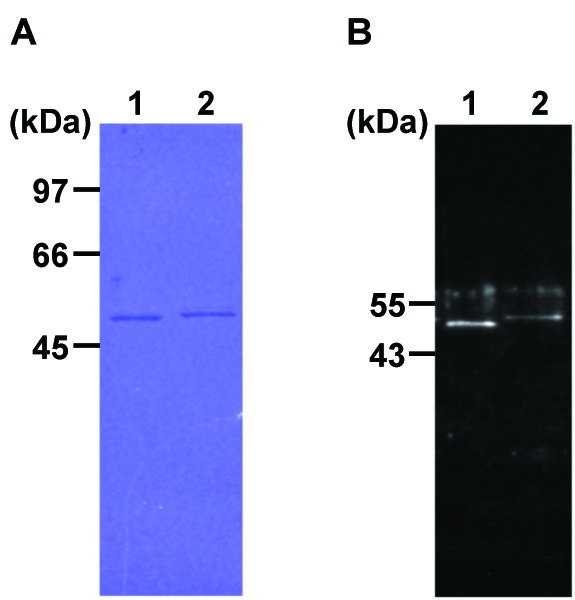
Figure 2. Purity of flagellins in flagellar filaments purified from A. avenae N1141 and K1 strains by SDS-PAGE (A) and Western blot analysis (B). A. The proteins were visualized by Coomassie brilliant blue R-250 (CBB) staining. Lane 1, flagellin of N1141 strain (pellet of 20,000 x g); lane 2, flagellin of K1 strain (pellet of 20,000 x g ). Molecular masses of the N1141 and K1 flagellins are 50,820 Da and 51,254 Da, respectively. B. The flagellins were detected by anti-flagellin antibody. Lane 1, flagellin of N1141 strain (pellet of 20,000 x g); lane 2, flagellin of K1 strain (pellet of 20,000 x g).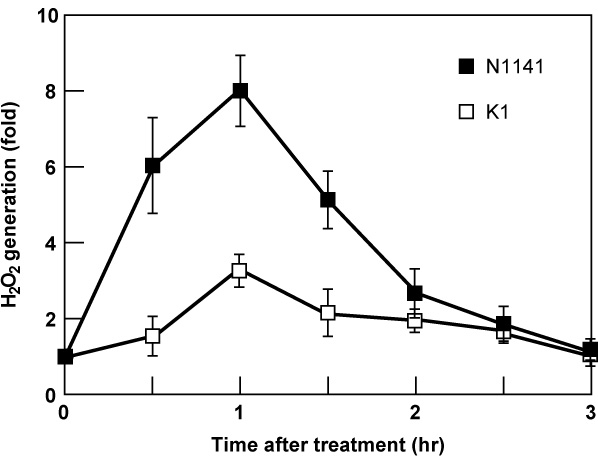
Figure 3. Induction of H2O2 generation in cultured rice cells by flagellins purified from A. avenae. Time course of H2O2 generation in cultured rice cells treated with 100 nM flagellins from the avirulent N1141 strain (solid squares) or virulent K1 strain (open squares). The Y axis represents fold change relative to amount of H2O2 in cultured cells before treatment. Bars indicate the standard deviation of the mean of three experiments.
Recipes
- Skimmed milk solution
10% skim milk powder
80 mM sodium hydrogen L(+)-glutamate monohydrate
Sterilize the solution by autoclaving - A. avenae skimmed milk stock solution
Culture A. avenae on LB solid medium for 24 h at 30 °C
Add 1 ml of skimmed milk solution to the culture solid medium, and harvest
Freeze immediately in liquid nitrogen, and store at -80 °C - 25 mM TBS buffer (pH 7.4)
25 mM 2-amino-2-hydroxymethyl-1,3-propanediol
137 mM NaCl
2.68 mM KCl
Sterilize the solution using a sterile filter (0.22 µm) - 20x R2 Major solution
800 mM KNO3
50.7 mM (NH4)2SO4
20.3 mM MgSO4·7H2O
20.4 mM CaCl2·2H2O
35 mM NaH2PO4·2H2O
Sterilize the solution using a sterile filter (0.22 µm) - 1,000x R2 Minor solution
7.17 mM MnSO4·5H2O
7.65 mM ZnSO4·7H2O
0.5 mM CuSO4·5H2O
48.52 mM H3BO3
0.52 mM Na2MoO4·2H2O
Sterilize the solution using a sterile filter (0.22 µm) - 500x Fe liquid solution
10.07 mM Na2·EDTA
9.89 mM FeSO4·7H2O
Sterilize the solution using a sterile filter (0.22 µm) - 1,000x MS vitamin solution (10 ml)
1.03 g MS vitamin powder (1,000x)
Sterilize the solution using a sterile filter (0.22 µm) - 2,4-dichlorophenoxyacetic acid solution
181 mM 2, 4-dichlorophenoxyacetic acid
Dissolve 2, 4-dichlorophenoxyacetic acid in1 ml of dimethyl sulfoxide - R2S medium (pH 5.6) (1,000 ml)
50 ml 20x R2 major solution
1 ml 1,000x R2 minor solution
2 ml 500x Fe liquid solution
1 ml 1,000x vitamin solution
0.1 ml 2, 4-dichlorophenoxyacetic acid solution
30 g sucrose
Adjust pH to 5.6 with KOH. Fill up to 1,000 ml with distilled water, and sterilize by autoclaving. - 2x sample buffer (pH 6.8)
125 mM Tris
139 mM SDS
12% (v/v) 3-mercapto-1,2-propanediol
20% (v/v) glycerol
Adjust pH to 6.8 with HCl, and add to 1% BPB - Electrophoresis buffer
3.5 mM SDS
24.8 mM Tris
192 mM glycine - CBB staining solution
0.25% (w/v) CBB
50% (v/v) methanol
5% (v/v) acetic acid - Destaining solution
50% (v/v) methanol
10% (v/v) acetic acid - Transfer buffer
25 mM Tris
192 mM glycine
1.7 mM SDS
20% (v/v) methanol - TBST buffer
25 mM Tris
13.7 mM NaCl
0.1% (v/v) Tween-20 - Blocking buffer
5% (w/v) skim milk powder
Dissolve skim milk powder in TBST buffer - 50 mM potassium phosphate buffer (pH 7.9)
50 mM KH2PO4
Adjust pH to 7.9 with KOH - Luminol solution
1.1 mM luminol
Dissolve luminol in 50 mM potassium phosphate buffer (pH 7.9) - Potassium ferricyanide solution
14 mM potassium ferricyanide
Dissolve potassium ferricyanide in 50 mM potassium phosphate buffer (pH 7.9)
Acknowledgments
This protocol was adapted from the previously published studies (Che et al., 2000; Hirai et al., 2011; Katsuragi et al., 2015; Schwacke and Hager, 1992). This work was supported by Technology of Japan and the Program for Promotion of Basic and Applied Research for Innovations in Bio-Oriented Industry and Grant-in-Aid for Scientific Research (B) (25292067) from the Ministry of Education, Culture, Sports, Science.
References
- Che, F. S., Nakajima, Y., Tanaka, N., Iwano, M., Yoshida, T., Takayama, S., Kadota, I. and Isogai, A. (2000). Flagellin from an incompatible strain of Pseudomonas avenae induces a resistance response in cultured rice cells. J Biol Chem 275(41): 32347-32356.
- Hirai, H., Takai, R., Iwano, M., Nakai, M., Kondo, M., Takayama, S., Isogai, A. and Che, F. S. (2011). Glycosylation regulates specific induction of rice immune responses by Acidovorax avenae flagellin. J Biol Chem 286(29): 25519-25530.
- Katsuragi, Y., Takai, R., Furukawa, T., Hirai, H., Morimoto, T., Katayama, T., Murakami, T. and Che, F. S. (2015). CD2-1, the C-terminal region of flagellin, modulates the induction of immune responses in rice. Mol Plant Microbe Interact 28(6): 648-658.
- Schwacke, R. and Hager, A. (1992). Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca(2+) and protein-kinase activity. Planta 187(1): 136-141.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hirai, H., Furukawa, T., Katsuragi, Y. and Che, F. (2016). Purification of Flagellin from Acidovorax avenae and Analysis of Plant Immune Responses Induced by the Purified Flagellin. Bio-protocol 6(16): e1898. DOI: 10.21769/BioProtoc.1898.
Category
Biochemistry > Protein > Immunodetection
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link