- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vivo DCs Depletion with Diphtheria Toxin and MARCO+/MOMA1+ Cells Depletion with Clodronate Liposomes in B6.CD11c-DTR Mice
Published: Vol 6, Iss 15, Aug 5, 2016 DOI: 10.21769/BioProtoc.1885 Views: 11698
Reviewed by: Pinchas TsukermanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In Vitro Bone Marrow–Derived Dendritic Cells (BMDC) Generation for Antigen Presentation Assay
Sudhakar Singh [...] Sharvan Sehrawat
Apr 20, 2025 4366 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3995 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3553 Views
Abstract
To evaluate precisely the relative roles of different splenic phagocytic cells during an immune response, efficient methods for the depletion of specific populations are needed. Here, we describe the protocols for the depletion of splenic dendritic cells (DCs) by human diphtheria toxin (DTx) treatment in target mice (which express the human DTx receptor in all CD11c+ DCs) and for the specific depletion of MARCO+/MOMA-1+ marginal zone macrophages (MZMΦs) with clodronate liposomes (ClLip) treatment (when a small dose of ClLip is ministered, MZMΦs preferentially uptake ClLip, and clodronate is released inside those cells causing apoptosis-mediated cell death). These protocols are adaptations from previous works (Jung et al., 2002; McGaha et al., 2011), and were used to evaluate the respective roles of DCs and of MZMΦs during the acute phase of experimental blood-stage malaria infection (Borges da Silva et al., 2015).
Materials and Reagents
- 25 gauge needle (BD Biosciences, catalog number: 305122 )
- Cell strainer (100 μm pore size) (Corning Incorporated, catalog number: 352360 )
- 15 ml tubes (TPP, catalog number: 91015 )
- 1 ml syringes (BD Biosciences, catalog number: 309659 )
- 1 ml syringes with 30 gauge needle (BD Biosciences, catalog number: 328278 )
- B6 mice (Jackson Laboratories, model: B6 )
- B6.CD11c-DTR mice (Jackson Laboratories, model: C57BL/6 )
- Diphtheria toxin (Sigma-Aldrich, catalog number: D0564 )
- Sodium clodronate (Melone Pharmaceutical, catalog number: 22560-50-5 )
- RPMI 1640 (Thermo Fisher Scientific, catalog number: 11875093 )
- Fetal bovine serum heat inactivated (Thermo Fisher Scientific, catalog number: 10437028 )
- Penicillin-Streptomycin (Thermo Fisher Scientific, catalog number: 15140122 )
- L-glutamine (Thermo Fisher Scientific, catalog number: 25030081 )
- Sodium pyruvate (Thermo Fisher Scientific, catalog number: 11360070 )
- 2-mercaptoethanol Thermo Fisher Scientific, catalog number: 21985023 )
- Halothane (Sigma-Aldrich, catalog number: H0150000 )
- Monoclonal antibody (mAb) to MARCO (R&D Systems, catalog number: FAB2956P )
- mAb to MOMA-1 (Abcam, catalog number: ab51814 )
- mAb to F4/80 (eBioscience, catalog number: 47-4801 )
- mAb to CD11b (BD Biosciences, catalog number: 562950 )
- mAb to CD11c (eBiosciences, catalog number: 17011482 )
- mAb to I-Ab (eBiosciences, catalog number: 46532082 )
- NaCl
- KCl
- Na2HPO4
- KH2PO4
- 1x phosphate buffered saline (PBS) (see Recipes)
- Staining Buffer (see Recipes)
- Supplemented RPMI 1640 (see Recipes)
- ClLip (see Recipes)
Equipment
- Centrifuge (Eppendorf, model: 5804 )
- Laminar flow hood (AirClean Systems, catalog number: AC8000HLF )
- FACSCanto II Flow Cytometer, 8-color, lasers blue/red/violet (BD Biosciences, catalog number: 338962 )
- Push-Pull syringe pump (KD Scientific, Model: KDS120 )
Procedure
- DCs depletion in B6.CD11c-DTR mice
- Inject B6.CD11c-DTR mice (6-8 weeks age) i.p. with 2 ng/g of body weight of DTx (200 μl volume per mouse), or with PBS as depletion control (200 μl volume per mouse), using a syringe with 25 gauge needle.
- 24 h after injection, mice are euthanized with halothane by inhalation (or other approved euthanasia protocol).
- The spleens are removed and processed in a cell strainer inside a sterile culture hood (with 5 ml of supplemented RPMI 1640), followed by two washes with RPMI (at 300 x g, 5 min, 4 °C).
- Splenocytes are then stained with mAbs (0.5 µl per 106 cells – each mAb at initial 0.5 mg/ml concentration, diluted in 25 µl of Staining Buffer) to CD11c and I-Ab (1 incubation period of 30 min), re-suspended in staining buffer (200 µl) and analyzed in a FACSCanto device, to evaluate depletion efficiency.
- Inject B6.CD11c-DTR mice (6-8 weeks age) i.p. with 2 ng/g of body weight of DTx (200 μl volume per mouse), or with PBS as depletion control (200 μl volume per mouse), using a syringe with 25 gauge needle.
- MZMΦs depletion in B6 mice with ClLip
- Inject B6 mice (6-8 weeks age) i.v. with 8.5 µg/g of body weight of ClLip (200 µl per mice) prepared as described in (van Rooijen et al., 1993), or with PBS-loaded liposomes (200 µl per mice, 8.5 µg/g of body weight) as depletion control, using a syringe with 30 gauge needle.
- 24 h to seven days after injection, mice are euthanized with halothane by inhalation (or other approved euthanasia protocol) and bled by cardiac puncture with a 1 ml syringe with a 25 gauge needle.
Note: In both time points only MZMΦs are depleted in our protocol (this was done as a control to ensure only MZMΦs are depleted in our protocol, opposite to injection of higher concentrations of ClLip). - The spleens are removed and processed in a cell strainer inside a sterile culture hood (with 5 ml of supplemented RPMI 1640), followed by two washes with RPMI (at 300 x g, 5 min, 4 °C).
- Splenocytes are then stained with mAbs (0.5 µl per 106 cells – each mAb at initial 0.5 mg/ml concentration, diluted in 25 µl of Staining Buffer) to MARCO, MOMA-1, CD11b, CD11c and I-Ab (1 incubation period of 30 min), re-suspended in Staining Buffer (200 µl) and analyzed in a FACSCanto device, to evaluate depletion efficiency. An example of results obtained with this experiment is shown in Figure 1A and 1B, respectively.
Note: Different DC subsets might have different sensitivity to DTx treatment, with different repopulation rates following initial depletion. Thus, for prolonged experiments, an important step would be to perform repopulation rate assays for the DCs subsets of interest.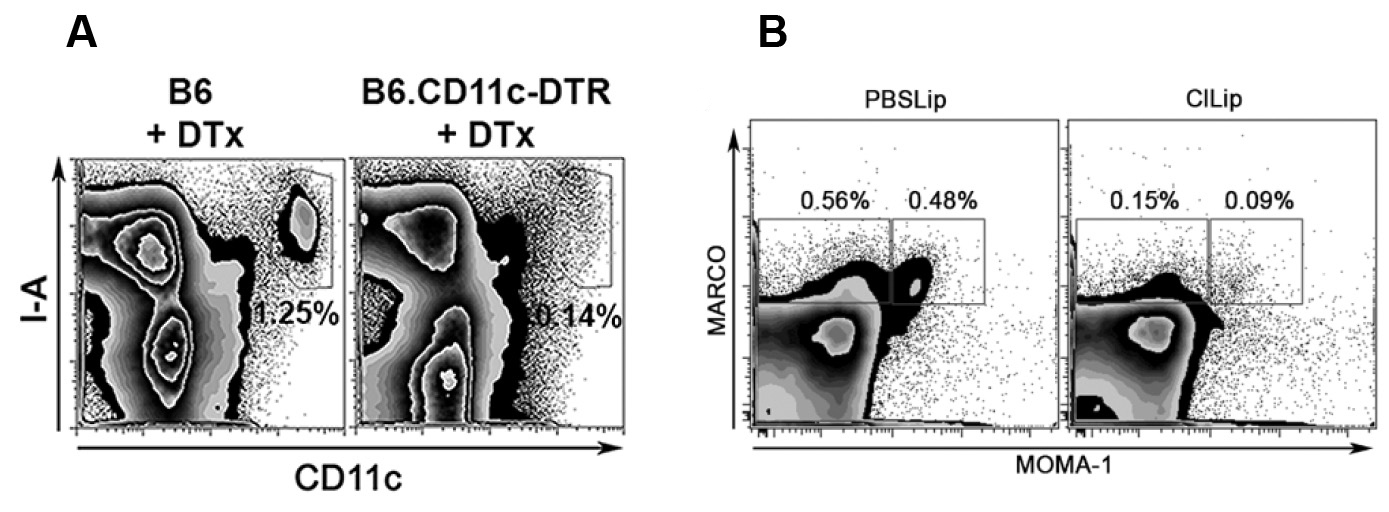
Figure 1. In vivo depletion of splenic DCs and MZMΦs. A. Representative plots of mice depleted of DCs using as controls of depletion B6 mice treated with DTX (here, CD11c-DTR mice treated with DTx present with a lower percentage of CD11c+I-A+ DCs, as showed in the gate in the upper right of each histogram (this decrease in percentage is an indicative of cell depletion). Adapted from Borges da Silva et al. (2015). B. Representative plots of mice depleted of MZMΦs using as controls B6 mice treated with ClLip (here, ClLip-treated mice present with a lower percentage of MARCO+MOMA-1+ MZMΦs, as showed in the gates represented in each histogram (this decrease in percentage is an indicative of cell depletion). Adapted from Borges da Silva et al. (2015).
- Inject B6 mice (6-8 weeks age) i.v. with 8.5 µg/g of body weight of ClLip (200 µl per mice) prepared as described in (van Rooijen et al., 1993), or with PBS-loaded liposomes (200 µl per mice, 8.5 µg/g of body weight) as depletion control, using a syringe with 30 gauge needle.
Recipes
- 1x phosphate buffered saline (PBS)
Dissolve the following in 800 ml distilled H2O
8 g NaCl
0.2 g KCl
1.44 g Na2HPO4
0.24 g KH2PO4
Adjust pH to 7.4
Adjust volume to 1 L with additional distilled H2O
Sterilize the solution
Note: Adjust the pH using HCl and NaOH. - Staining Buffer
Dissolve the following in 200 ml PBS
1 ml 10% azide
2 ml fetal bovine serum heat inactivated - Supplemented RPMI 1640
Dissolve the following in 200 ml RPMI 1640 medium
2 ml L-glutamine
20 ml fetal bovine serum heat inactivated
2 ml sodium pyruvate
2 ml Penicillin-Streptomycin
200 µl 2-mercaptoethanol - ClLip
- Inject (0.2 ml/min) an ethereal solution of 50 mg phosphatidylcholine and 8 mg cholesterol into 5 ml of a 50 mM/L clodronate aqueous solution maintained at 42 °C, by using a syringe adapted in a Push-Pull syringe pump, equipped with a fine-gauge needle (No 3D).
- During injection, a nitrogen stream will be infused into the clodronate solution, up to liposome formation and removal of residual solvent.
- Centrifuge liposome suspension (22,800 x g, 30 min, 25 °C). Wash twice with PBS, and resuspend in 2 ml PBS.
- Filter through a 0.8 Am polycarbonate membrane.
- Inject (0.2 ml/min) an ethereal solution of 50 mg phosphatidylcholine and 8 mg cholesterol into 5 ml of a 50 mM/L clodronate aqueous solution maintained at 42 °C, by using a syringe adapted in a Push-Pull syringe pump, equipped with a fine-gauge needle (No 3D).
Acknowledgments
HBdS was supported by an award from FAPESP (number: 2014/00810-5) and MRDL was supported by a grant from FAPESP (number: 2013/07140-2), and from CNPq 303676/2014-0 (MRDL) and 448765/2014-4 (MRDL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The DTX-mediated depletion of DCs was adapted from Jung et al. (2002), and the ClLip-mediated depletion of MZMΦs was adapted from McGaha et al. (2011).
References
- Borges da Silva, H., Fonseca, R., Cassado Ados, A., Machado de Salles, E., de Menezes, M. N., Langhorne, J., Perez, K. R., Cuccovia, I. M., Ryffel, B., Barreto, V. M., Marinho, C. R., Boscardin, S. B., Alvarez, J. M., D'Imperio-Lima, M. R. and Tadokoro, C. E. (2015). In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria. PLoS Pathog 11(2): e1004598.
- Jung, S., Unutmaz, D., Wong, P., Sano, G., De los Santos, K., Sparwasser, T., Wu, S., Vuthoori, S., Ko, K., Zavala, F., Pamer, E. G., Littman, D. R. and Lang, R. A. (2002). In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17(2): 211-220.
- McGaha, T. L., Chen, Y., Ravishankar, B., van Rooijen, N. and Karlsson, M. C. (2011). Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood 117(20): 5403-5412.
- van Rooijen, N. and van Kesteren-Hendrikx, E. (2003). "In vivo" depletion of macrophages by liposome-mediated "suicide". Methods Enzymol 373: 3-16.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Silva, H. B. D., Tadokoro, C. E. and D’Império-Lima, M. R. (2016). In vivo DCs Depletion with Diphtheria Toxin and MARCO+/MOMA1+ Cells Depletion with Clodronate Liposomes in B6.CD11c-DTR Mice. Bio-protocol 6(15): e1885. DOI: 10.21769/BioProtoc.1885.
- Borges da Silva, H., Fonseca, R., Cassado Ados, A., Machado de Salles, E., de Menezes, M. N., Langhorne, J., Perez, K. R., Cuccovia, I. M., Ryffel, B., Barreto, V. M., Marinho, C. R., Boscardin, S. B., Alvarez, J. M., D'Imperio-Lima, M. R. and Tadokoro, C. E. (2015). In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria. PLoS Pathog 11(2): e1004598.
Category
Immunology > Animal model > Mouse
Immunology > Immune cell function > Dendritic cell
Immunology > Immune cell function > Macrophage
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










