- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cell Tracer Violet and CellTracker Red CMTPX Staining of Purified Mature Plasmodium-infected Red Blood Cells
Published: Vol 6, Iss 15, Aug 5, 2016 DOI: 10.21769/BioProtoc.1884 Views: 11324
Reviewed by: Pinchas TsukermanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Efficient staining methods to identify Plasmodium-infected red blood cells (iRBCs) are crucial to discriminate precisely the immune cells responsible for their elimination from circulation. Here, we describe the protocol for the purification of iRBCs and their subsequent staining with the vital dyes Cell Tracer Violet (CTV) or CellTracker Red CMTPX (CMTPX), both of which readily diffuse into cells and bind covalently to intracellular amines. The iRBCs stained by using this protocol were used in ex vivo phagocytosis assays, to determine the ability of splenic dendritic cells of phagocytizing these parasites (Borges da Silva et al., 2015).
Materials and Reagents
- 15 ml tubes (TPP, catalog number: 91015 )
- 1 ml syringes (BD Biosciences, catalog number: 309659 )
- B6.Rag2-/- (RAGKO) mice (Jackson Laboratories, C57BL/6)
- Plasmodium chabaudi (AS strain)
- RPMI 1640 (Thermo Fisher Scientific, catalog number: 11875093 )
- Fetal bovine serum heat inactivated (Thermo Fisher Scientific, catalog number: 10437028 )
- Penicillin-Streptomycin (Thermo Fisher Scientific, catalog number: 15140122 )
- L-glutamine (Thermo Fisher Scientific, catalog number: 25030081 )
- Sodium pyruvate (Thermo Fisher Scientific, catalog number: 11360070 )
- 2-mercaptoethanol (Thermo Fisher Scientific, catalog number: 21985023 )
- Cell Tracer Violet (CTV) (Thermo Fisher Scientific, catalog number: C34557 )
- CellTracker Red CMTPX (CMTPX) (Thermo Fisher Scientific, catalog number: C34552 )
- Halothane (Sigma-Aldrich, catalog number: H0150000 )
- NaCl
- KCl
- Na2HPO4
- KH2PO4
- Percoll (GE Healthcare Dharmacon, catalog number: 17089101 )
- 1x phosphate buffered saline (PBS) (see Recipes)
- 74% Percoll (see Recipes)
Equipment
- Centrifuge (Eppendorf, model: 5804 )
- 1-ml micropipettes
- 25 gauge needle (BD Biosciences, catalog number: 305122 )
- Laminar flow hood (AirClean Systems, catalog number: AC8000HLF )
- Epi-Fluorescent microscope (Leica Microsystems, model: DM6000B )
Procedure
- 6-8 weeks old RAG2KO mice (used here because we observed higher proportion of mature parasite forms in circulation instead bound to endothelial cells-personal observation) are infected i.p. with 106 Pc-infected iRBCs. The infection in RAGKO allows the posterior collection of a greater proportion of mature iRBCs (Borges da Silva, personal observation).
- Seven days after infection, mice are euthanized with halothane by inhalation and bled by cardiac puncture with a 1 ml syringe with a 25 gauge needle, in the presence of approximately 50 µl of heparin. A volume of approximately 500 µl of blood is enough to collect up to 5 x 108 mature iRBCs.
Note: Verify the institution approved ethical regulations on animal welfare if other euthanization methods are in place. - The blood collected is diluted in approximately 1 ml of PBS 1x (1:1), and placed on a 74% Percoll solution in 15 ml tubes (5 ml). The tubes are centrifuged for 30 min at 2,500 x g, at room temperature with acceleration/break of 5/0, respectively.
- After the centrifugation, the mature iRBCs are located as a layer above the 74% Percoll (Figure 1), and are collected by using 1-ml micropipettes into new 15 ml tubes. The mature iRBCs are then washed twice with 1x PBS.
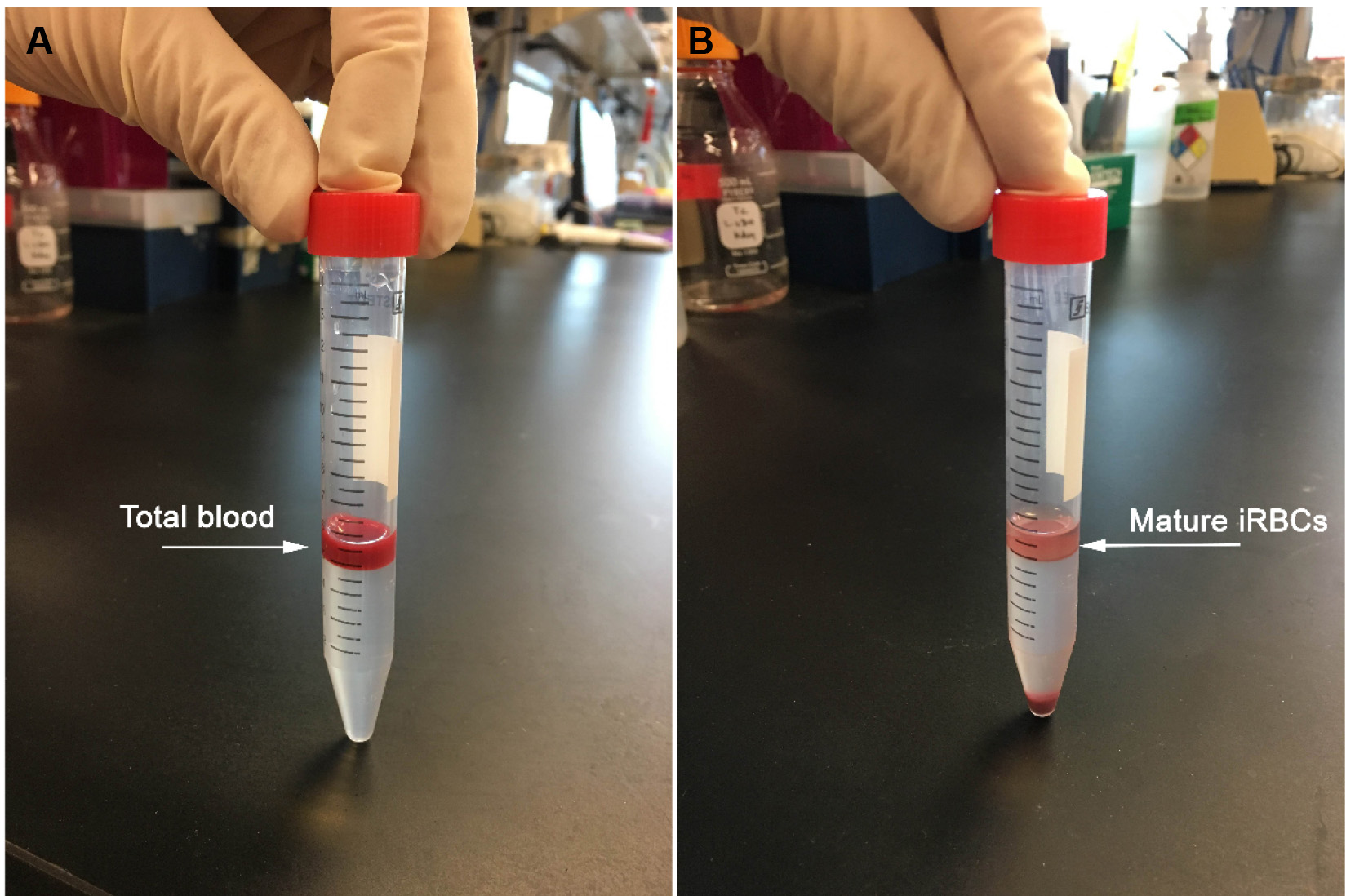
Figure 1. Percoll-based purification of mature iRBCs. A. A representative picture of 15-ml conical tubes containing RBCs over Percoll 74% before centrifugation. B. A representative picture of 15-ml conical tubes containing the layer with mature iRBCs after Percoll 74% centrifugation. - After washing, a sample of the mature iRBCs (approximately 5 µl) is collected for a blood smear, to confirm the purity for mature iRBCs versus non-mature iRBCs (Figure 2) by counting the percentage of mature iRBCs versus non-mature iRBCs in the blood smear (at this point, it is expected up to 97% of mature iRBCs in the blood smear). Then, 1 x 108 mature iRBCs (resuspended in 2 ml of PBS) are incubated with 5 µM of CTV or with 5 µM of CMTPX (both dyes previously diluted in DMSO and with final suspension volume of 2 ml), at 37 °C, for 30 min.
Notes:- The expected purity for the samples is between 90-99% of mature iRBCs.
- During the incubation, mix the cells gently for homogenization.
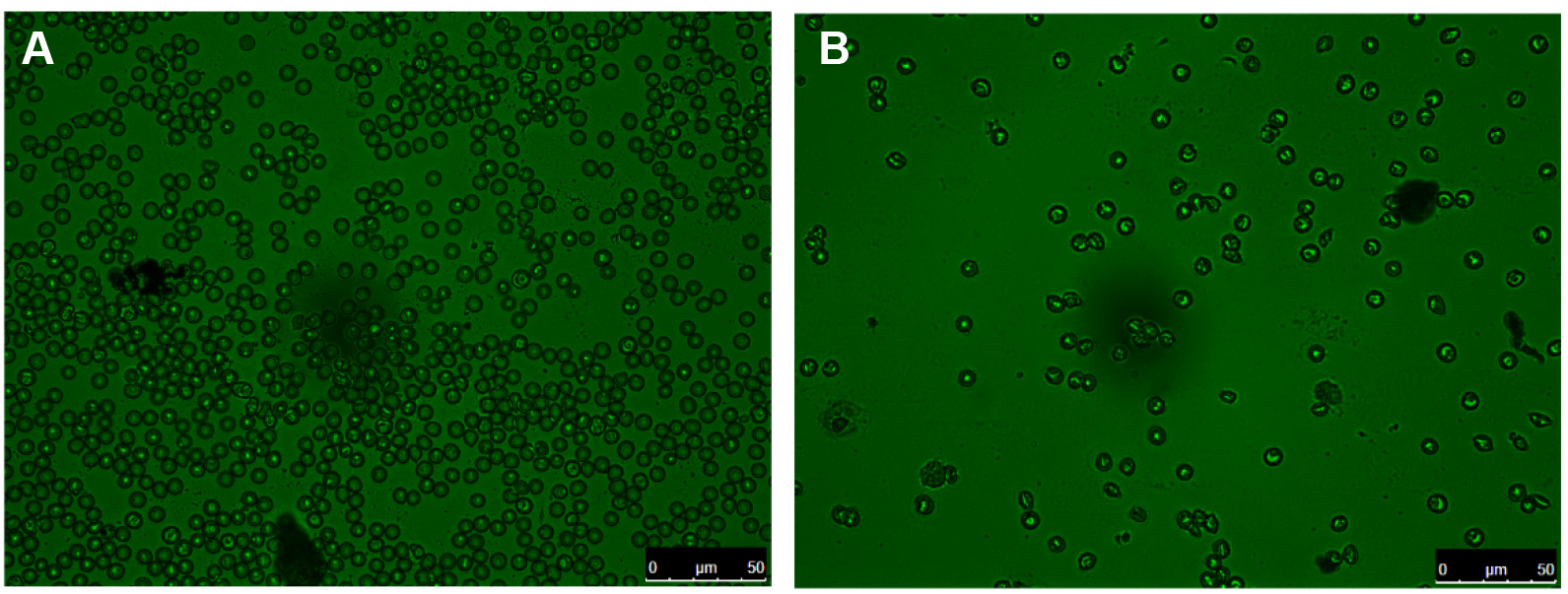
Figure 2. Mature iRBCs purification assessment. A. A representative picture of a blood smear of a Plasmodium-infected mouse, pre-Percoll enrichment. B. A representative picture of a blood smear of post-Percoll enrichment blood of the same mice. Obs: The infected RBCs can be detected by the bright green spots (these images were acquired in Bright Field, in a Leica DM6000B Epi-Fluorescent Microscope). - The expected purity for the samples is between 90-99% of mature iRBCs.
- After incubation, the mature iRBCs are washed twice with 1x PBS for removal of excess dye. The stained mature iRBCs are, then, ready for use in phagocytosis assays as described in (Borges da Silva et al., 2015).
Notes
- This procedure usually yields between 95-97% of mature iRBCs purity. However, when done with mice with lower parasitemia levels, there is a drop in these numbers up to 50% purity. Thus, it is preferential to collect blood from RAG2KO mice with > 50% parasitemia (verified upon a blood smear previously done to select mice).
Recipes
- 1x phosphate buffered saline (PBS)
Dissolve the following in 800 ml distilled H2O
8 g NaCl
0.2 g KCl
1.44g Na2HPO4
0.24 g KH2PO4
Adjust pH to 7.4
Adjust volume to 1 L with additional distilled H2O
Sterilize the solution
Note: Adjust the pH using HCl and NaOH. - 74% Percoll
Percoll is commercially available at a 10x concentration. To make a 1x concentrated Percoll solution (which is equivalent to a 100% Percoll solution), it is necessary to dilute 9 parts of Percoll (9 ml GE Healthcare) with 1 part of 10x PBS (1 ml).
To make 74% Percoll, it is necessary to dilute 3.7 ml of 100% Percoll with 1.3 ml of 1x PBS.
Acknowledgments
HBdS was supported by an award from FAPESP (number: 2014/008105) and MRDL was supported by a grant from FAPESP (number: 2013/071402), and from CNPq 303676/20140 (MRDL) and 448765/20144 (MRDL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Borges da Silva, H., Fonseca, R., Cassado Ados, A., Machado de Salles, E., de Menezes, M. N., Langhorne, J., Perez, K. R., Cuccovia, I. M., Ryffel, B., Barreto, V. M., Marinho, C. R., Boscardin, S. B., Alvarez, J. M., D'Imperio-Lima, M. R. and Tadokoro, C. E. (2015). In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria. PLoS Pathog 11(2): e1004598.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Silva, H. B. D., Tadokoro, C. E. and D’Império-Lima, M. R. (2016). Cell Tracer Violet and CellTracker Red CMTPX Staining of Purified Mature Plasmodium-infected Red Blood Cells. Bio-protocol 6(15): e1884. DOI: 10.21769/BioProtoc.1884.
- Borges da Silva, H., Fonseca, R., Cassado Ados, A., Machado de Salles, E., de Menezes, M. N., Langhorne, J., Perez, K. R., Cuccovia, I. M., Ryffel, B., Barreto, V. M., Marinho, C. R., Boscardin, S. B., Alvarez, J. M., D'Imperio-Lima, M. R. and Tadokoro, C. E. (2015). In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria. PLoS Pathog 11(2): e1004598.
Category
Cell Biology > Cell isolation and culture > Cell isolation
Immunology > Immune cell function > Dendritic cell
Immunology > Immune cell staining > Immunodetection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









