- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Intra- and Extracellular Glucose in Mycelium of Fusarium oxysporum
Published: Vol 6, Iss 14, Jul 20, 2016 DOI: 10.21769/BioProtoc.1868 Views: 10891
Reviewed by: Zhaohui LiuShahin S. AliSusheel KumarAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Steady-state and Flux-based Trehalose Estimation as an Indicator of Carbon Flow from Gluconeogenesis or Glycolysis
Ritu Gupta and Sunil Laxman
Jan 5, 2020 5531 Views

Slot Blot Analysis of Intracellular Glyceraldehyde-Derived Advanced Glycation End Products Using a Novel Lysis Buffer and Polyvinylidene Difluoride Membrane
Takanobu Takata [...] Togen Masauji
Jul 20, 2024 1840 Views

Quantifying Intestinal Glucose Absorption Using Isolated Vascularly Perfused Rat Small Intestine
Cecilie Bæch-Laursen [...] Jens Juul Holst
Nov 20, 2025 1584 Views
Abstract
To study alterations in the metabolism and/or in the transport of glucose during Fusarium oxysporum vegetative growth, we determined intracellular glucose levels in different fungal strains, as well as the amount of glucose remaining in the supernatants after growth in synthetic medium (SM) supplemented with either 0.05 or 2.5% glucose. We used the Glucose (GO) Assay Kit (Sigma-Aldrich) following the instructions of the manufacturer with some modifications. The protocol described here can be applied to other filamentous fungi.
Keywords: Glucose determinationMaterials and Reagents
- Monodur nylon filters 15 µm diameter (Filtravibracion S.L., catalog number: Nylon-15 )
- 2 ml Eppendorf tubes
- Sterile plastic funnels (80 mm diameter) (Tecnylab, catalog number: 45000150 )
- 5 mm-diameter glass beads (Sigma-Aldrich, catalog number: 18406-500G )
- Microtiter plates (TermoFisher Scientific, catalog number: 2205 )
- Fusarium oxysporum f.sp. lycopersici microconida suspensions from wild type and Δcon7-1 mutant strains
- Sterile dH2O
- Potato dextrose broth medium (PDB) (Scharlau, catalog number: 01483 )
- Glycerol (Merck, catalog number: 104092 )
- Glucose (Coger SAS, catalog number: 24379.363 )
- Na2CO3 (Sigma-Aldrich, catalog number: S2127 )
- Glucose (GO) Assay Kit (Sigma-Aldrich, catalog number: GAGO-20 )
GO Assay includes: - Glucose Oxidase/Peroxidase Reagent (Sigma-Aldrich, catalog number: G3660 )
- o-Dianisidine Reagent (Sigma-Aldrich, catalog number: D2679 )
- Glucose Standard Solution (Sigma-Aldrich, catalog number: G3285 )
- H2SO4, ACS reagent (Merck, catalog number: 108131.1000 )
- MgSO4·7H2O (Merck, catalog number: 1058865.5000 )
- KH2PO4 (Merck, catalog number: 104873.1000 )
- KCl (Merck, catalog number: 104933.0500 )
- NH4NO3 (Merck, catalog number: 101187.1000 )
- FeSO4 (Merck, catalog number: 103965.0500 )
- ZnSO4·7H2O (Merck, catalog number: 108883 )
- MnSO4·H2O (Merck, catalog number: 105941 )
- 0.1% benzoic acid
- Extraction buffer (see Recipes)
- Synthetic medium (SM) (see Recipes)
- H2SO4 (12 N) (see Recipes)
Equipment
- Mini-BeadBeater -16 homogenizer (BioSpec Products)
- Orbital incubator (Infors, Multitron Pro)
- Sterile spatula (Fisher Scientific, catalog number: S50821 )
- Microcentrifuge (Fisher Scientific, EppendorfTM MiniSpin plusTM)
- Hemocytometer (Thoma) (Marienfeld, catalog number: 06 407 10 )
- Fluorimeter (TECAN, SpectraFluorPlus, model: F129005 )
- Freeze-dryer (Virtis, model: BT4KZL-105 )
- Water bath (Selecta, model: 6000138 )
- Vortex (IKA, model: MS2 Mini shaker )
Procedure
- F. oxysporum f.sp. lycopersici wild type strain 4287 (race 2) was obtained from J. Tello, Universidad de Almeria, Spain. Δcon7-1 mutant, lacking a transcription factor essential for morphogenesis and virulence, was described previously (Ruiz-Roldan et al., 2015). Strains are stored at -80 °C with 30% glycerol as microconidial suspension.
- For fresh microconidia production, aliquots of frozen microconidial stocks are inoculated into 100 ml of potato dextrose broth (PDB) and incubated for 3 days at 150 rpm and 28 °C in an orbital incubator. Cultures are then filtered through Monodur nylon filters placed on funnels to separate mycelia (Figure 1A), and centrifuged at 3,020 x g for 5 min to collect microconidia. Finally, pellets containing fresh microconidia are resuspended into 1 ml sterile dH2O and counted using a hemocytometer.
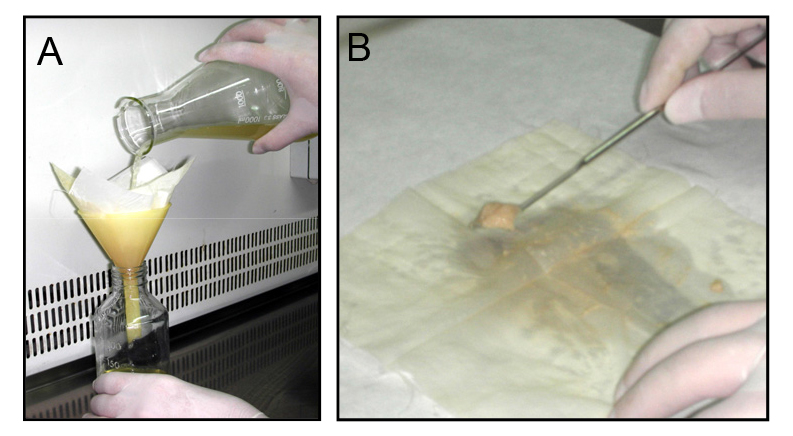
Figure 1. Filtration and mycelia harvesting procedure. A. Filtration of a fungal culture through a Monodur nylon membrane placed on a funnel. B. Subsequent harvesting of mycelia by scraping a nylon membrane using a spatula. - Aliquots containing 4 x 108 freshly obtained microconidia are inoculated into 100 ml of SM containing either 0.05 or 2.5% glucose and grown at 170 rpm and 28 °C for 24 h.
- After harvesting by Monodur filtration, mycelia are washed twice with sterile dH2O, separated from the filter by scraping using a spatula (Figure 1B) and finally lyophilized.
- Mycelia dry-weight is determined using a precision weighing scale.
Note: Normally, the amount of dry mycelia obtained from 100 ml cultures varies between 5 and 10 µg. - After dry-weight determination, samples are introduced into 2 ml-Eppendorf tubes containing a 5 mm-diameter glass bead and disrupted by 3 cycles of 30 sec agitation each using a Mini-BeadBeater homogenizer. Samples are then resuspended into 250 μl of 0.25 M Na2CO3 and incubated at 95 °C on a water bath for 4 h.
- The supernatant is harvested by centrifugation at 5,000 x g for 3 min.
- The amount of glucose remaining in culture supernatants from the different strains is also determined.
- Glucose levels are calculated from glucose standard curves and referred to mycelium dry-weight in each strain. Aliquots (40 μl each) are used for quantification of intracellular glucose levels using the glucose determination kit GAGO (https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Bulletin/gago20bul.pdf) following these instructions of the manufacturer with some modifications (indicated in underlines).
Note: The assay was repeated three times with independent biological samples. - Reagent Preparation
- Glucose Oxidase/Peroxidase Reagent. Store the unopened kit reagent at 2-8 °C. Each capsule contains 500 units of glucose oxidase (Aspergillus niger), 100 purpurogallin units of peroxidase (horseradish) and buffer salts. Dissolve the contents of the capsule in an amber bottle with 39.2 ml of deionized water. The solution is stable up to one month at 2-8 °C and for at least 6 months frozen at -20 °C. Discard if turbidity develops.
- o-Dianisidine Reagent. Store the unopened kit reagent at 2-8 °C. Minimize exposure to light. The preweighed vial contains 5 mg of o-dianisidine dihydrochloride. Reconstitute the contents of the o-dianisidine vial with 1.0 ml of deionized water. Invert the vial several times to dissolve. Avoid exposing the reagent to light. Solution is stable for 3 months at 2-8 °C.
- Prepare the Assay Reagent by adding 0.8 ml of the o-Dianisidine Reagent to the amber bottle containing the 39.2 ml of Glucose Oxidase/Peroxidase Reagent. Invert bottle several times to mix. Minimize exposure to light. Solution is stable up to 1 month at 2-8 °C. Discard if turbidity develops or color forms.
- Glucose Standard Solution. D-Glucose, 1.0 mg/ml in 0.1% benzoic acid. This standard is traceable to a NIST standard and is supplied ready-to-use. It is stable at 2-8 °C for at least six months. Discard if turbidity develops.
- Standard curve
- Prepare low-concentration D-Glucose standard solutions by diluting the 1.0 mg/ml stock solution in dH2O to final concentrations ranging from 0 (reagent blank) to 80 μg/ml.
- Pipette 40 µl aliquots of diluted D-Glucose standard solutions into a microtiter plate.
- Start the reaction by adding 80 µl of Assay Reagent to each well and mixing.
- Incubate exactly 30 min at 37 °C. Stop the reaction by adding 80 µl of 12 N H2SO4 into each well. Mix thoroughly by pipetting several times.
- Measure the absorbance against thereagent blank at 540 nm.
- Plot Absorbance at 540 nm (Y-axis) vs. µg of glucose (X-axis) (Figure 2). If the standard curve is not linear, results will be inaccurate. R-values of around 0.99 are normally obtained with this kit.
- Sample analysis
- Follow the directions given in Standard Curve, substituting D-Glucose standard solutions for 40 µl fungal experimental samples diluted 250 times.
Note: Depending on the sample, it might be necessary to optimize the dilution factor. - Calculate the amount of D-glucose in the experimental samples from the standard curve (Figure 2).
- Multiply the mg glucose determined above by the dilution factor made in sample preparation (x 250).
- Express mg of glucose in µg and divide it by the amount of dry mycelium in each case expressed in µg.
Representative data
- Figure 2 shows a representative standard curve for calculation of glucose concentration.
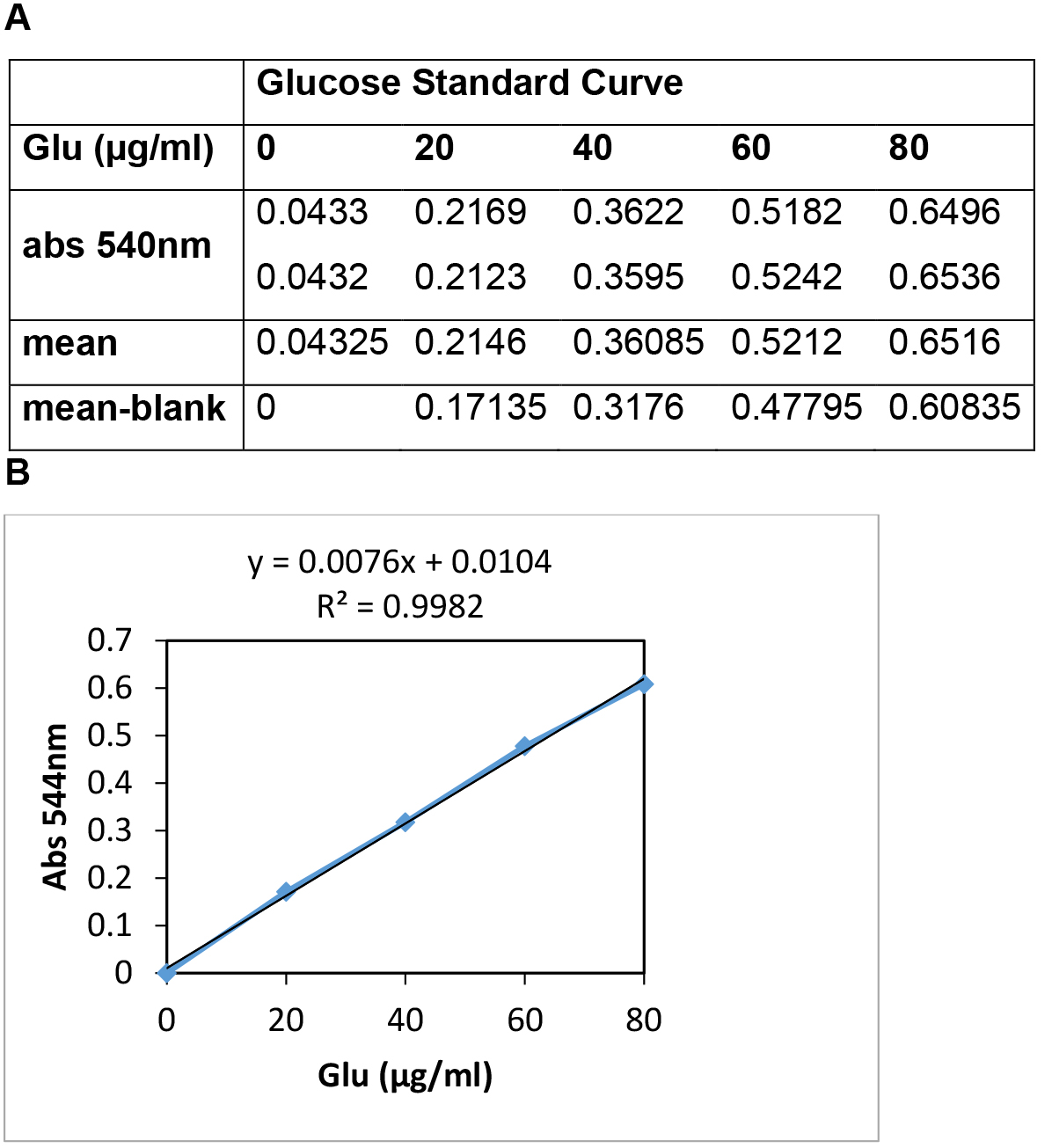
Figure 2. A representative standard curve for calculation of glucose concentration. A. Representative Absorbance values at 540 nm of the diluted D-Glucose standard solutions. B. Graphical representation of Absorbance values (Y-axis) vs. µg of glucose of the standards (X-axis).
In this example, an Absorbance value at 540 nm of 0.35 indicates that our test sample contains: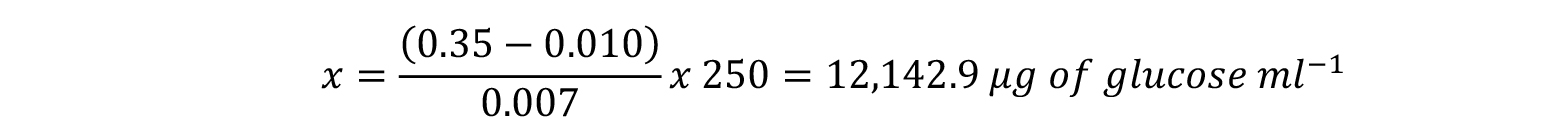
- Figure 3 shows a representative example of data obtained following this protocol.
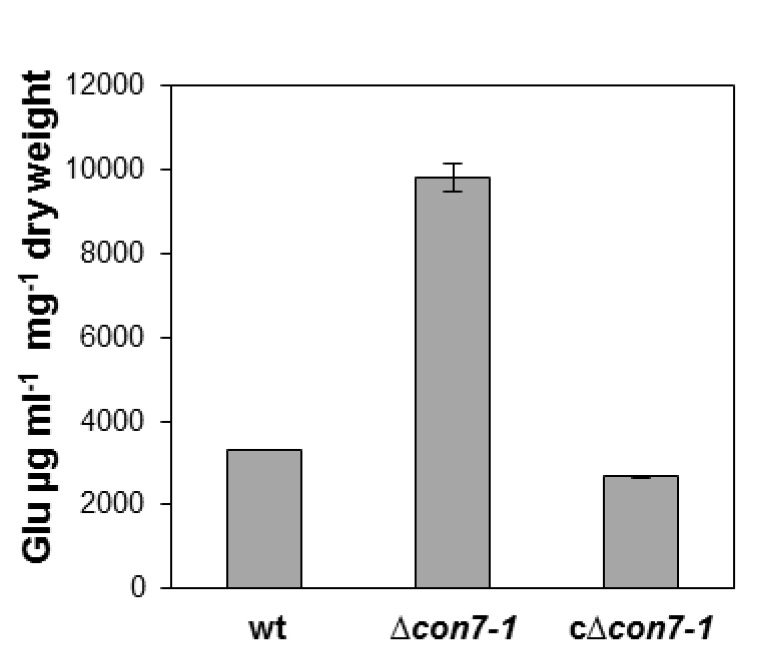
Figure 3. Extracellular glucose in culture supernatants of different fungal strains after 24 h growth in synthetic medium containing 2.5% glucose calculated using the Glucose determination kit GAGO. Bars represent standard errors calculated from three independent experiments with three replicates each.
Recipes
- Extraction buffer
Dissolve 0.25 M Na2CO3 in deionized water. - Synthetic medium (SM)
0.2 g/L MgSO4·7H2O
0.2 g/L KH2PO4
0.2 g/L KCl
1 g/L NH4NO3
0.01 g/L FeSO4
0.01 g/L ZnSO4·7H2O
0.01 g/L MnSO4·H2O
10 g/L glucose
Dissolve in deionized water an sterilize by autoclaving - H2SO4 (12 N)
Add 16.7 ml of 36 N stock solution to 33.3 ml deionized water
Acknowledgments
This research was supported by Junta de Andalucia (Proyecto de Excelencia CVI-7319) and the Spanish Ministerio de Economia y Competitividad (grant BIO2013-47870 and the Ramon y Cajal Program). This protocol was adapted from Ruiz-Roldan et al., 2015.
References
- Bergmeyer, H. U. and Bernt, E. (1974). Methods of enzymatic analysis. 2nd edition. New York, NY: 1205-1212.
- Di Pietro, A. and Roncero, M. I. (1998). Cloning, expression, and role in pathogenicity of pg1 encoding the major extracellular endopolygalacturonase of the vascular wilt pathogen Fusarium oxysporum. Mol Plant Microbe Interact 11(2): 91-98.
- Ruiz-Roldan, C., Pareja-Jaime, Y., Gonzalez-Reyes, J. A. and Roncero M. I. (2015). The Transcription factor Con7-1 Is a master regulator of morphogenesis and virulence in Fusarium oxysporum. Mol Plant Microbe Interact 28(1): 55-68.
- Xu, H. J., Xue, J., Lu, B., Zhang, X. C., Zhuo, J. C., He, S. F., Ma, X. F., Jiang, Y. Q., Fan, H. W., Xu, J. Y., Ye, Y. X., Pan, P. L., Li, Q., Bao, Y. Y., Nijhout, H. F. and Zhang, C. X. (2015). Two insulin receptors determine alternative wing morphs in planthoppers. Nature 519(7544): 464-467.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ruiz-Roldan, C. and Roncero, M. I. G. (2016). Determination of Intra- and Extracellular Glucose in Mycelium of Fusarium oxysporum. Bio-protocol 6(14): e1868. DOI: 10.21769/BioProtoc.1868.
Category
Biochemistry > Carbohydrate > Glucose
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link