- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Flavonoids from Piper delineatum Leaves by Chromatographic Techniques
Published: Vol 6, Iss 14, Jul 20, 2016 DOI: 10.21769/BioProtoc.1867 Views: 18603
Reviewed by: Arsalan DaudiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Heterologous Production of Artemisinin in Physcomitrium patens by Direct in vivo Assembly of Multiple DNA Fragments
Nur Kusaira Khairul Ikram [...] Henrik Toft Simonsen
Jul 20, 2023 2333 Views

Utilizing FRET-based Biosensors to Measure Cellular Phosphate Levels in Mycorrhizal Roots of Brachypodium distachyon
Shiqi Zhang [...] Maria J. Harrison
Jan 20, 2025 2388 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1181 Views
Abstract
The genus Piper (Piperaceae) is widely distributed in the tropical and subtropical regions of the world, and species belonging to this genus are included in the Ayurvedic system of medicine and in folklore medicine of Latin America. Phytochemical investigations of Piper species have led to the isolation of several classes of physiologically active compounds such as alkaloids, amides, pyrones, dihydrochalcones, flavonoids, phenylpropanoids, lignans and neolignans. In an ongoing investigation of bioactive secondary metabolites from Piper species, herein, we describe the isolation procedure of nine flavonoids, including two chalcones and two flavanones from the leaves of Piper delineatum Trel. (Piperaceae), a shrub native to tropical regions of the Americas. All compounds were elucidated by spectroscopic and spectrometric methods, and comparison with data reported in the literature.
Keywords: PiperMaterials and Reagents
- Silica gel 60 (particle size 63-200 mm) (Macherey-Nagel, catalog number: 815330 )
- Silica gel 60 (particle size 15-40 mm) (Macherey-Nagel, catalog number: 815650 )
- TLC silica gel 60 F254 plates (Merck Millipore Corporation, catalog number: 105735 )
- SIL G/UV254 (20 x 20 cm), Macherey-Nagel, catalog number: 805023 )
- Pre-coated TLC-plates UV254 20 x 20 cm (Macherey-Nagel, catalog number: 821030 )
- Round-bottom tubes (40 ml, 200 x 20 mm) (Simax, Alamo, catalog number: 00684800 )
- Round-bottom tubes (8 ml, 120 x 12 mm) (Simax, Alamo, catalog number: 00684400 )
- Microcapillary tube 1-5 μl (Sigma-Aldrich, catalog number: P0549-1PAK )
- NMR simple tubes 5 mm (Wilmad® LabGlass, catalog number: 528-PP-7 )
- Leaves of the plant species (Piper delineatum) at the mature stage were collected in Iquitos, Maynas Province, Department of Loreto, Perú in November 2009. A voucher specimen (10484) was identified by botanist Juan Ruiz Macedo and was deposited at the Amazonense Herbarium of Universidad Nacional de la Amazonia Peruana, Iquitos, Perú.
- Ethanol absolute (Panreac, catalog number: 141086 )
- Dichloromethane (CH2Cl) (Panreac, catalog number: 131254 )
- Ethyl acetate (Panreac, catalog number: 141318.1212 )
- Hexanes (Panreac, catalog number: 121347.1612 )
- Chloroform (Panreac, catalog number: 161252 )
- Diethyl ether (Panreac, catalog number: 142770.0311 )
- Acetone (Panreac, catalog number: 131007.1212 )
- Methanol (Panreac, catalog number: 141091.1211 )
- Isopropanol (Panreac, catalog number: 131090 )
- Acetone deuterated [(CD3)2CO] (Sigma-Aldrich, catalog number: 151793-25G )
- Magnesium sulfate anhydrous (Panreac, catalog number: 212486 )
- Sulfuric acid (Panreac, catalog number: 141058 )
- Acetic acid glacial (Panreac, catalog number: 141008 )
- Sea sand washed thin grain QP (Panreac, catalog number: 211160.0416 )
- Sephadex LH-20 (Sigma-Aldrich, catalog number: LH20100 )
- Oleum: Acetic acid-water-sulfuric acid (20:4:1) (see Recipes)
- Coating rotor with sorbent for the CPTLC (4 mm thickness) (see Recipes)
Equipment
- Rotary cutter mill (Mateu and Sole, S.L., Constructores, MATSO, model: B-2, nº 258 )
- Soxhlet extraction apparatus (ANORSA, catalog number: 322060 )
- Rotary R-210 evaporator (Sigma- Aldrich, Büchi® Rotavapor®, catalog number: Z565466 )
- Separating funnel, 2 L (Sigma-Aldrich, catalog number: Z330663 )
- Erlenmeyer flasks (100 ml and 500 ml) (Duran, catalog numbers: Z232793-1EA and Z232831-1EA , respectively)
- Teflon funnel holder
- Solvent-pouring funnel (stem L, O.D. 0.65 x 22 mm) (Sigma-Aldrich, catalog number: 548804 )
- Evaporating flask (pear-shaped, 1L) (Sigma-Aldrich, catalog number: Z402990 )
- Round-bottom flask (5 L) (Sigma-Aldrich, catalog number: Z302872 )
- Glass Pasteur pipets 225 mm (BRAND, catalog number: 747720 )
- Pasteur pipette rubber bulbs, 2 ml (Sigma-Aldrich, catalog number: Z111597-12EA )
- Heating bath (Sigma-Aldrich, catalog number: Z563544 )
- Heating mantle 5 L (Selecta, catalog number: 3031450 )
- Hot plate (Selecta, catalog number: 1000443 )
- Liebig Condenser (Sigma-Aldrich, catalog number: Z531006 )
- Glass chromatography column (CC) for silica gel (50 x 9 cm) (Fisher Scientific, catalog number: 12058880 )
- Glass chromatography column (CC) for Sephadex (60 x 4.5 cm) (Fisher Scientific, catalog number: 12011550 ).
- Centrifugal preparative thin layer chromatography system (CPTLC) (Chromatotron, Harrison Research Inc., model: 7924T )
- Silica gel PF254 disks (Merck Millipore Corporation, catalog number: 107749 2500 )
- TLC Chamber rectangular for TLC plates (7.5 x 15.5 x 8.0 cm) (Sigma-Aldrich, catalog number: Z204226 )
- TLC Chamber rectangular for PTLC plates (27.0 x 26.5 x 7.0 cm) (Sigma-Aldrich, catalog number: Z126195-1EA )
- Glass atomizer reagent sprayer (125 ml) (Sigma-Aldrich, catalog number: Z529737-1EA )
- Glass vacuum filter (Fisher Scientific, catalog number: 11979659 )
- Micro-spatula (8 in) (Sigma-Aldrich, catalog number: Z513342-1PAK )
- Analytical balance, semi-micro balance (Fisher Scientific, Mettler ToledoTM, catalog number: 11142062 ; model: NewClassic MS105DU)
- Nuclear Magnetic Resonance (NMR) Spectrometers (Bruker, model: Bruker Avance 400 ; Bruker Avance 500 ).
- Optical rotations polarimeter (CHCl3 at 25 °C) (PerkinElmer, model: 241 automatic polarimeter )
- Ultraviolet (UV) spectrophotometer (JASCO, model: V-560 )
- Infrared (IR) spectrophotometer (Bruker, model: IFS 55 , IFS 28/55 )
- High resolution electron impact (HREI) mass spectrometer (MS) (MasSpec, model: Micromass VG Autospec magnetic sector , M series)
Procedure
Note: The whole procedure described below takes around 6 months to complete.
- Finely grind the air-dried leaves of Piper delineatum (252.2 g) into small pieces using a rotary cutter mill, and extract with ethanol (4 L) in a Soxhlet apparatus (Figure 1) for 24 h at around 80 °C.
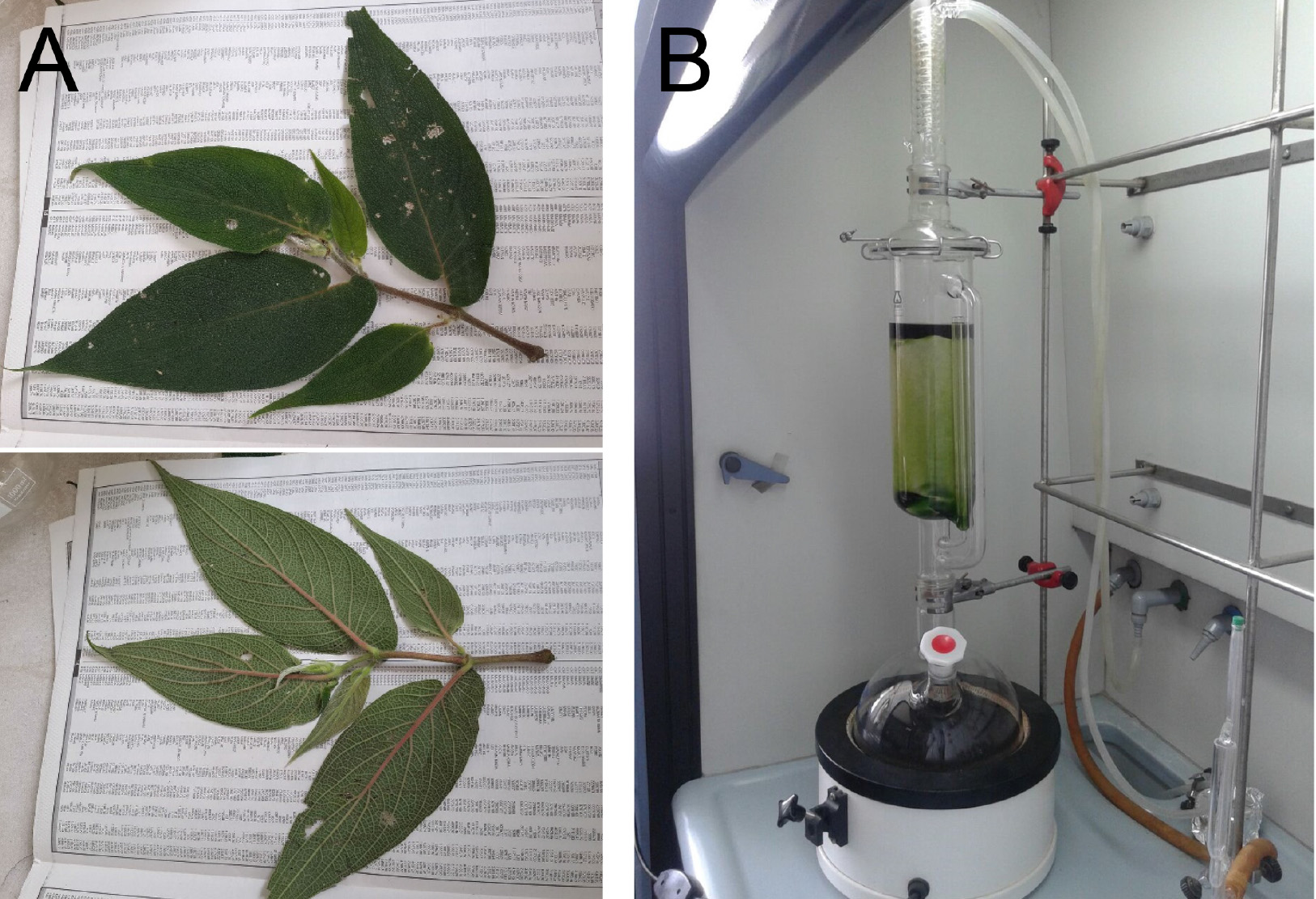
Figure 1. Plant material and extraction procedure. A. Piper spp. leaves. B. Soxhlet apparatus used for the extraction of the plant material. - Evaporate the solvent under reduced pressure in a rotary evaporator (at ≤ 50 °C, around 1.30 h) provided 57.3 g (22.7%) of crude extract.
- Re-suspend the crude extract in 750 ml of water and slowly pour into a separating funnel (2 L) placed onto a metallic stand (Figure 2).
- Add 750 ml of CH2Cl2 to the separating funnel, and hand-shake gently several times. Then, allow the solvent to settle into immiscible phases and recover both phases, the organic phase (bottom layer), and the aqueous phase (top layer) into separate Erlenmeyer flasks.
- Place the aqueous phase inside the separating funnel and add 750 ml of CH2Cl2 and repeat the operation twice.
- Combine the organic phases obtained in the three extractions, and add magnesium sulfate anhydrous (10 g) to remove the residual water. Swirl the solution. If the drying agent is clumped together, add more until it looks free-flowing. Filter, through a filter funnel, the total organic phase and collect the filtrate into a 1 L evaporating flask (pear-shaped).

Figure 2. Liquid-liquid partition of the crude extract using a separating funnel - Re-suspend the crude extract in 750 ml of water and slowly pour into a separating funnel (2 L) placed onto a metallic stand (Figure 2).
- Afterwards, the organic phase is taken to dryness by removal of the CH2Cl2 under vacuum in a rotary evaporator (at ≤ 40 °C) (Figure 3), yielding 30 g (11.9%) of organic fraction (dark green solid).

Figure 3. Rotary evaporator to remove solvent - Pack a column chromatography (CC) (50 x 9 cm) by placing 1.5 cm layer of fine sand and then silica gel (particle size 63-200 mm, 430 g) to a depth of 20 cm, next cover with another 1.5 cm layer of sand to protect the adsorbent surface. Allow an appropriate volume of hexanes (4 x 1 L) to pass through the CC to encourage bubbles to rise and the silica gel to settle so that it packs tightly into the column.
- Dissolve the organic fraction in 40 ml CH2Cl2, mix with silica gel until it forms a slurry, and remove the solvent under the rotary evaporator until a dry powder is obtained.
- Spread the dried powder extract, slowly and uniformly, on the upper top of the packed column (Figure 4), and carry out the elution by gradient, using mixtures of hexanes/EtOAc of increasing polarity as eluent (10:0 to 0:10, starting with 100% hexanes, followed by hexanes/EtOAc mixtures 8:2, 6:4, 4:6, 2:8, 1:9 and finishing with 100% ethyl acetate, 1 L each solvent mixture).
- Collect the resulting fractions (15 fractions, 400 ml each) in round-bottomed flasks (1 L).
- Subsequently, remove the mobile phase from each fraction in a rotary evaporator (at ≤ 4 °C), and dissolve the residue in 20 ml CH2Cl2 and transfer to round-bottomed tubes (40 ml).

Figure 4. Column chromatography (CC) - Dissolve the organic fraction in 40 ml CH2Cl2, mix with silica gel until it forms a slurry, and remove the solvent under the rotary evaporator until a dry powder is obtained.
- Combine these 15 fractions into 10 subfractions (A-J) based on their similarities TLC profiles (Figure 5).
- To run the TLC plates, add the solvent mixtures of hexanes/ethyl acetate of increasing polarity, starting from 8:2 (8 ml hexanes plus 2 ml ethyl acetate) and finishing with 6:4, into the TLC chamber to a depth of just under 0.5 cm.
- Apply an aliquot of each fraction, using a microcapillary, to the baseline (marked about 0.7 cm from one end of the plate) of the TLC (around 7 x 5 cm for TLC) and place into the TLC chamber.
- Develop the TLC, leaving the solvent to rise up the TLC plate by capillary action, until the solvent reaches almost the top (0.5 cm of the plate).
- Then, visualize the developed TLC under a UV lamp using 254 nm wavelengths, and stained with oleum system and heat the plates to 80-100 °C in a hotplate until spots appear.

Figure 5. Collected fractions from CC and analysis by TLC. A. Collected fractions in round-bottom tubes from the column chromatography showed in Figure 4. B. Visualization of thin layer chromatography (TLC) developed plates of the collected fractions that are further combined into 10 subfractions based on their TLC profiles. - To run the TLC plates, add the solvent mixtures of hexanes/ethyl acetate of increasing polarity, starting from 8:2 (8 ml hexanes plus 2 ml ethyl acetate) and finishing with 6:4, into the TLC chamber to a depth of just under 0.5 cm.
- 1H NMR analysis reveals that fractions C (hexanes/EtOAc, 8:2), H (hexanes/EtOAc, 3:7) and J (EtOAc) are rich in flavonoids. The solvent used for the NMR experiments is deuterated acetone, (CD3)2CO.
Note: To prepare the NMR sample: dissolve the sample in 0.6 ml of (CD3)2CO, filter through Pasteur pipette, equipped with a filter paper, and discharge into the NMR tube. - Chromatograph fraction C (2.2 g) on a column chromatography (60 x 4.5 cm) packed with Sephadex LH-20 (hexanes/CHCl3/MeOH, 2:1:1 as eluent). Dissolve fraction C in the minimum volume of eluent (hexanes/CHCl3/MeOH, 2:1:1), and load to the top of the column. Develop the column using 1 L of eluent, and collect fractions of 30 ml in round-bottom tubes (40 ml), and combine by TLC profile into five fractions (C1-C5).
Notes: - Before loading the sample for column chromatography on Sephadex, pass 1.5 L of a mixture of mobile phase (hexanes/CHCl3/MeOH, 2:1:1) through the column to stabilize the Sephadex LH-20.
- Before loading the sample on Sephadex column chromatography, filter the sample through filter paper.
- Chromatograph fraction C3 (328.8 mg) by CPTLC, a preparative centrifugally accelerated radial thin-layer chromatograph (Figure 6).
- Apply the sample dissolved in a small volume of CH2Cl2 (3 ml), near the center of a spinning disk coated with a thin layer of sorbent (coating rotor, 4 mm thickness).
- The mobile phase is supplied by a Teflon funnel holder about 55 cm above bench level, and the solvent flows down onto the circular plate through a capillary tube in the funnel. Elution by mixtures of hexanes/diethyl ether (8:2, 7:3, 6:4 and 1:1, 100 ml each solvent mixture) forms circular bands of the separated components which are spun off from the edge of the rotor together with solvent.
- Collect 48 fractions into round-bottom tubes (8 ml).
- Combine fractions based on their similarities in TLC profiles, into eleven fractions (C3A-C3K).

Figure 6. Centrifugal preparative thin layer chromatography (CPTLC, Chromatotron) - Apply the sample dissolved in a small volume of CH2Cl2 (3 ml), near the center of a spinning disk coated with a thin layer of sorbent (coating rotor, 4 mm thickness).
- Dissolve subfractions C3E (16.4 mg) and C3H (17.7 mg) in Cl2CH2 (2 ml), and further purify by PTLC (plate 20 x 20 cm, 2.5 mm SiO2 thickness) (Figure 7, steps A-E).
- Load the sample as a band, as narrow as possible, at the baseline of the plate (marked about 2 cm from one end of the plate), with a Pasteur pipette (step A).
- Dry the plate, place in the chamber with the selected eluent (hexanes/EtOAc, 8:2 for subfraction C3E, and hexanes/CH2Cl2, 8:2 for subfraction C3H, 100 ml of eluent) and develop it (step B).
- Remove the plate (step C) from the chamber and visualize under UV light marking with a pencil around the band corresponding to the compound.
- Use a microspatula to scrape the marked band, containing the product, off the plate onto a lengthwise folder piece of clean white paper (step D).
Note: It is very important to deposit a thin line of sample slowly and uniformly, without touching the pipet too much against the silica as this will scrape it. If the desired band is not separated enough, repeat runs with another elution. - Place the scraping into a glass vacuum filter (packed with around 2 cm silica gel) and flush with EtOAc (4 x 5 ml) into a round-bottomed flask (step E). Remove the solvent under reduced pressure in a rotary evaporator.
- Afterwards, dissolve the sample in (CD3)2CO (0.5 ml) and with a Pasteur pipette transfer to a NMR tube to perform the NMR experiments. In this way, the following compounds can be isolated and purified: 2'-hydroxy-3,4',6'-thoxychalchone (compound I, 9.4 mg, Rf 0.42) (Boumendjel et al., 2008) from subfraction C3E, and compounds 2',4'-dihydroxy-3,6'-dimethoxychalcone (compound II, 3.6 mg, Rf 0.38) (Aponte et al., 2010) and 2', 4'-dihydroxy-6'-methoxychalcone (compound III, 8.3 mg, Rf 0.36) from subfraction C3H.
- Load the sample as a band, as narrow as possible, at the baseline of the plate (marked about 2 cm from one end of the plate), with a Pasteur pipette (step A).
- Chromatograph fraction H (5.4 g) on Sephadex LH-20 (CHCl3/MeOH, 1:1, 500 ml) to afford fractions H1-H9, and collect them in round-bottom flasks (10 ml each), following the procedure described in step 7.
- Chromatograph fraction H6 (304.8 mg) by CPTLC (CH2Cl2/acetone of increasing polarity, 10:0 to 1:1, starting from 100% CH2Cl2 and finishing with a mixture of CH2Cl2/acetone 50%, 200 ml) to afford nine fractions (H6A-H6I), and collect in round-bottom flasks (10 ml each), using the same procedure as described in step 8.
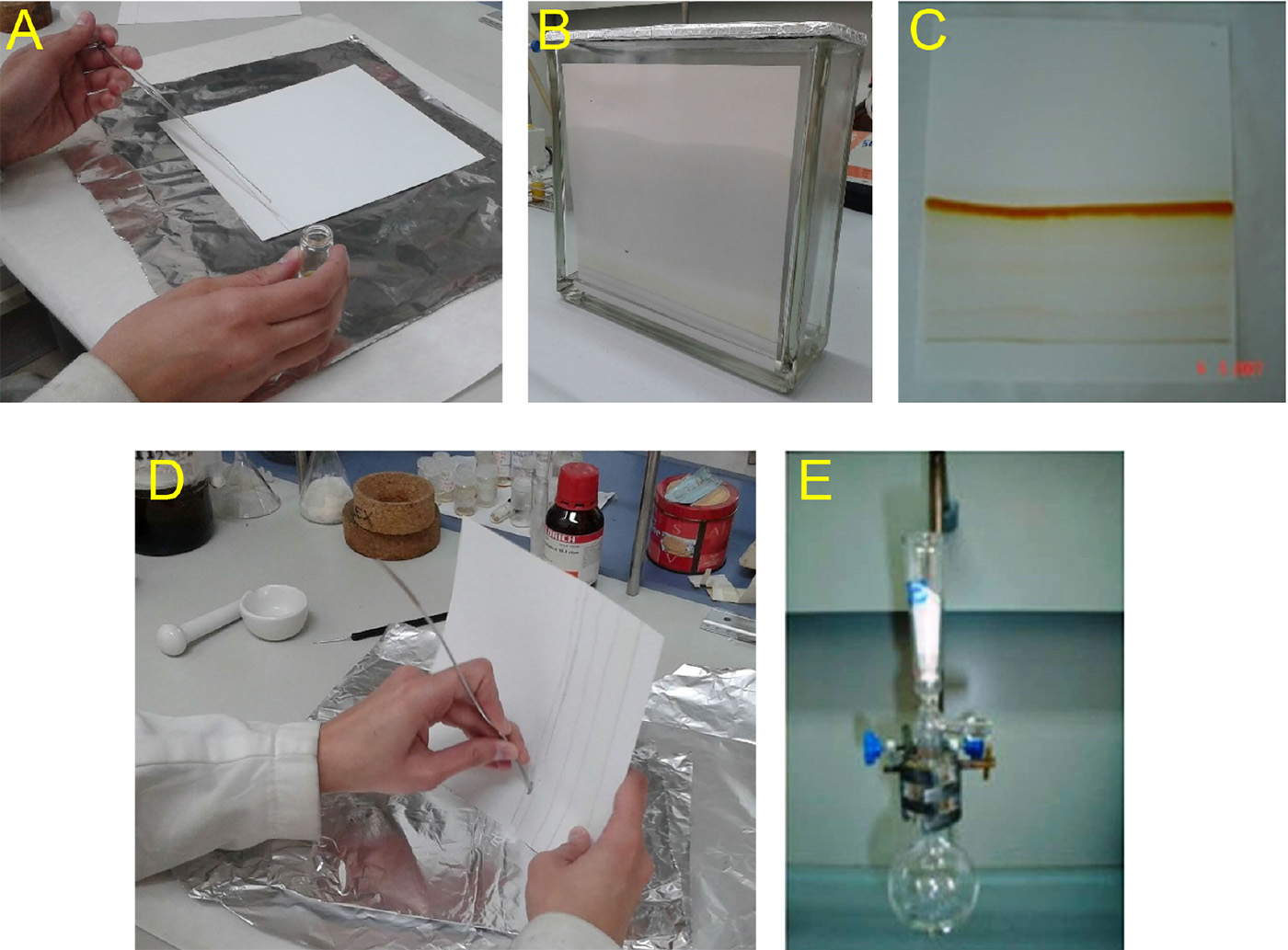
Figure 7. Steps by step guide of preparative thin layer chromatography (PTLC) procedure. A. Load the sample as a band at the plate. B. Place the PTLC in the chamber and develop the plate. C. Remove the plate and visualize it under UV light marking the band corresponding to the compound. D. Scrape the marked band. E. Place the scraping into a glass vacuum filter and flush with a solvent. - Further purify subfraction H6F (18.2 mg) by PTLC (hexanes/EtOAc, 3:2), following the same procedure described in step 9, and compounds 2',4'-dihydroxy-3,3',6'-trimethoxychalcone (compound IV, 4.7 mg, Rf 0.36) and 2',3'-dihydroxy-4',6'-dimethoxychalchone (compound V, 9.3 mg, Rf 0.40) (Chantrapromma et al., 2000) can be obtained.
- Chromatograph fraction H7 (593.0 mg) by CPTLC (CH2Cl2/acetone, 10:0 to 1:1) to give fractions H7A-H7G, following the same procedure as described in step 8.
- Subsequently, further purify subfraction H7F (23.1 mg) by PTLC (eluent: hexanes/isopropanol, 4:1) to yield compounds 2',3',5-trihydroxy-4',6',3-trimethoxychalcone (compound VI, 15.3 mg, Rf 0.47) and 2',4',4-trihydroxy-3,6'-dimethoxychalcone (compound VII, 3.8 mg, Rf 0.42) (Vogel et al., 2010,), following the same procedure described in step 9.
- Chromatograph fraction I (3.9 g) on Sephadex LH-20 (CHCl3/MeOH, 1:1) to afford fractions I1-I5. Further chromatograph fraction I3 (628.8 mg) by CPTLC (CH2Cl2/acetone, 10:0 to 7:3) to afford fractions I3A-I3L, following the same procedure described in step 8.
- Further purify subfractions I3F (16.4 mg) and I3H (21.2 mg) by PTLC (CH2Cl2/acetone, 9:1 and hexanes/isopropanol, 8:2, respectively) to afford compounds (-)-(2S)-8-hydroxy-5,7,3'-trimethoxyflavanone (compound VIII, 11.4 mg, Rf 0.44) and (-)-(2S)-7, 5'-dihydroxy-5,3'-dimethoxyflavanone (compound IX, 10.4 mg, Rf 0.39), respectively, following the same procedure described in step 9.
- In summary, the EtOH extract of leaves of P. delineatum was partitioned into a CH2Cl2/H2O solution. The CH2Cl2 fraction was subjected to multiple chromatographic steps to yield 9 flavonoids, whose structures were deduced by NMR and MS analysis (Figure 8).
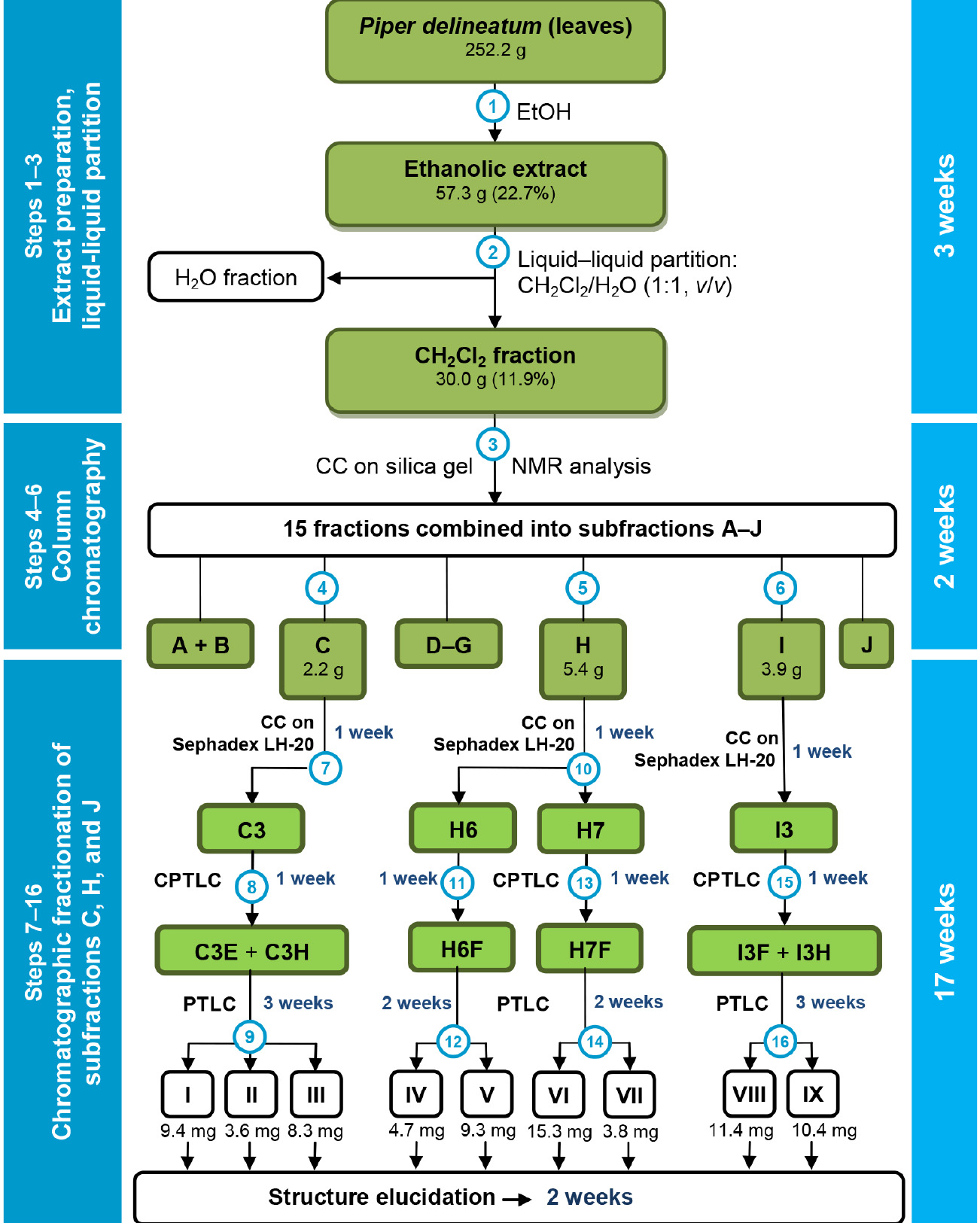
Figure 8. Flowchart for chromatographic steps to obtain flavonoids from leaves of Piper delineatum
Recipes
- Oleum: Acetic acid-water-sulfuric acid (20:4:1)
Mix 4 ml of sulfuric acid with 16 ml of water into an Erlenmeyer (200 ml), and slowly add acetic acid glacial (80 ml). Mix well and place in a glass atomizer sprayer. - Coating rotor with sorbent for the CPTLC (4 mm thickness)
Disk coated with a thin layer of silica gel (60 PF 254) TLC standard grade (75 g) with calcium sulfate hemihydrate (30 g, CaSO4, ½ H2O) sorbent and water (187 ml).
Acknowledgments
This protocol was adapted from previously published studies, Martín-Rodríguez et al. (2015). This work was supported by the European EU, FP7-REGPOT-2012-CT2012-316137-IMBRAIN project.
References
- Aponte, J. C., Castillo, D., Estevez, Y., Gonzalez, G., Arevalo, J., Hammond, G. B. and Sauvain, M. (2010). In vitro and in vivo anti-Leishmania activity of polysubstituted synthetic chalcones. Bioorg Med Chem Lett 20(1): 100-103.
- Boumendjel, A., Boccard, J., Carrupt, P. A., Nicolle, E., Blanc, M., Geze, A., Choisnard, L., Wouessidjewe, D., Matera, E. L. and Dumontet, C. (2008). Antimitotic and antiproliferative activities of chalcones: forward structure-activity relationship. J Med Chem 51(7): 2307-2310.
- Chantrapromma, K., Rat, A. p. Y., Karalai, C., Lojanapiwatana, V. and Seechamnanturakit, V. (2000). A chalcone and a dihydrochalcone from Uvaria dulcis. Phytochemistry 53(4): 511-513.
- Harwood, L. M. and Moody, C. J. (1994). Experimental organic chemistry: principles and practice. Blackwell Science.
- Martín-Rodríguez, A. J., Ticona, J. C., Jiménez, I. A., Flores, N., Fernández, J. J. and Bazzocchi, I. L. (2015). Flavonoids from Piper delineatum modulate quorum-sensing-regulated phenotypes in Vibrio harveyi. Phytochemistry 117: 98-106.
- Vogel, S., Barbic, M., Jurgenliemk, G. and Heilmann, J. (2010). Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur J Med Chem 45(6): 2206-2213.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bazzocchi, I. L., Ticona, J. C., Jiménez, I. A. and Flores, N. (2016). Isolation of Flavonoids from Piper delineatum Leaves by Chromatographic Techniques. Bio-protocol 6(14): e1867. DOI: 10.21769/BioProtoc.1867.
Category
Plant Science > Plant biochemistry > Other compound
Plant Science > Plant metabolism > Other compound
Biochemistry > Other compound > Flavonoid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link













