- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Denervation of Mouse Lower Hind Limb by Sciatic and Femoral Nerve Transection
Published: Vol 6, Iss 13, Jul 5, 2016 DOI: 10.21769/BioProtoc.1865 Views: 17475
Reviewed by: Oneil G. BhalalaAntoine de MorreeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Application of Electrical Stimulation to Enhance Axon Regeneration Following Peripheral Nerve Injury
Supriya S. Wariyar and Patricia J. Ward
Oct 5, 2023 2033 Views

Procedure for Reliable and Long-Lasting Ex Vivo Recordings of Sciatic Nerve Activity in Mice
Shani Berkowitz [...] Jérôme Joël Devaux
Mar 5, 2025 2554 Views

An Optimized Ex Vivo Protocol for Quantitative Electrophysiological Assessment of Neuromuscular Junctions and Skeletal Muscle Function Using the Aurora System
Can Cui [...] Wing Hoi Cheung
Jun 20, 2025 1951 Views
Abstract
The requirement and influence of the peripheral nervous system on tissue replacement in mammalian appendages remain largely undefined. Reports from salamander models of appendage regeneration (Singer, 1952; Singer, 1947; Kumar et al., 2007), and of human clinical skin and nail problems associated with spinal cord injury patients (Stover et al., 1994) suggest that appendage regeneration may have an important nerve component. To explore this question, we have generated hind limb tissues devoid of nerve supply. This protocol, combined with multi-color ‘Rainbow’ reporter mouse lines permits single cell clonal analysis and genetic lineage tracing studies in the absence of nerve supply (Rinkevich et al., 2014), exposing nerve requirements on cellular replacement and differentiation during tissue growth, maintenance, and regeneration.
Keywords: DenervationMaterials and Reagents
- Gauze pad
- Sterile drapes
- 4-0 plain gut suture, 18”, PS-4 needle (Ethicon)
- Handheld cautery: high temperature cautery fine tip (Bovie Medical, model: AA01 )
- Wild-type CD-1 mice (8-12 weeks old) (Charles River Laboratories International)
- Isoflurane (isothesia) (Henry Schein Animal Health)
- Veterinary ointment (Dechra, Puralube® Ophthalmic Ointment)
- 10% povidone iodine prep solution (Dynarex, catalog number: 1413 )
- 0.3 mg/ml buprenorphine (Buprenex) (Reckitt Benckiser)
Equipment
- Electric hair clippers
- Scissors
- CO2 chamber
- Far infrared warming pad (Kent Scientific)
Procedure
- Procedure description
- Ensure that adequate institutional animal care approvals are in place for the denervation procedure. Induce anesthesia with 2-3% inhaled isoflurane. Apply ophthalmic ointment to both eyes in order to prevent desiccation of the cornea during surgery.
- Use the toe pinch maneuver to ensure that the depth of anesthesia is adequate.
- Prior to starting the surgery, administer 0.1 mg/kg buprenorphine subcutaneously.
- Sciatic Nerve denervation
- Place the mouse in prone position on a clean, absorbent surface on top of a regulated heating pad.
- Remove hair from the posterior thigh and lower back of the mouse with electric clippers (Figure 1).
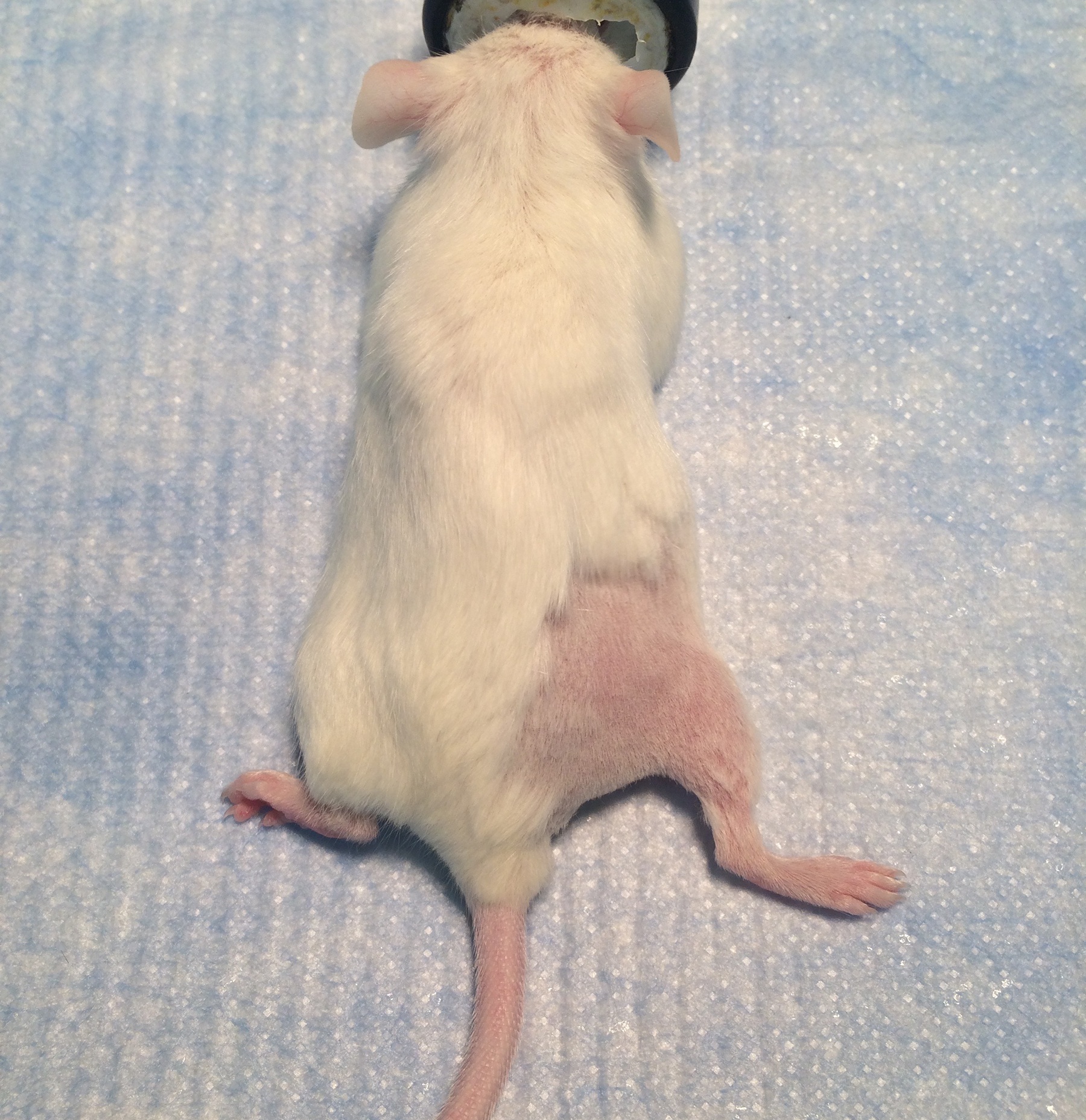
Figure 1. The posterior surgical site for sciatic nerve exposure. Shaving an area of adequate size prevents contamination of the surgical field. - Aseptically prepare the shaved skin with povidone iodine solution and drape with sterile drapes. Make sure to maintain sterile technique during the entire surgery. Antibiotics are not needed as long as the operative field remains sterile.
- Using sterile technique, make an incision parallel with the femur in the skin of the dorsal thigh. Use small sharp scissors to spread the tissue underneath the skin in order to mobilize the skin away from the underlying muscle. It is helpful to use self-securing skin hooks to open the operative field.
- The femur will be visible within the muscle (Figure 2; arrowheads indicate the femur.). Using the sharp scissors, carefully divide the muscle parallel to and just inferior to the femur. This will reveal the sciatic nerve. (Figure 3; arrowheads indicate the sciatic nerve.). The skin hooks can be inserted into the muscle to improve exposure of the nerve.

Figure 2. Exposure of the femur. The sciatic nerve is not initially visible but is located inferior and deep to the surface of the femur seen here.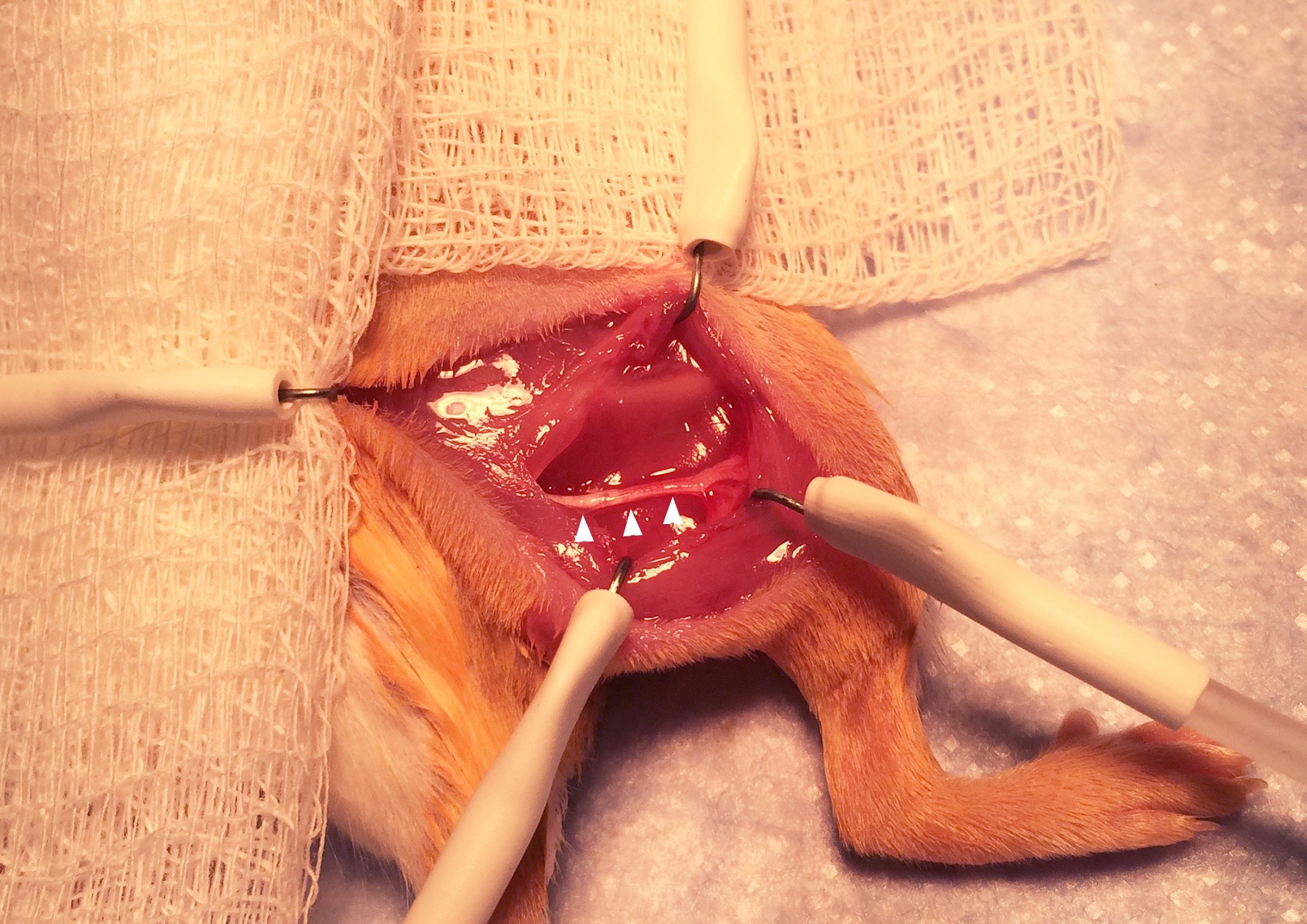
Figure 3. Exposure of the sciatic nerve. Exposing the nerve requires delicate dissection with fine scissors. - Obtain denervation by cutting away the 5 mm section of the sciatic nerve that is in the center of the operative field (Figure 4; P, proximal end. D, distal end). Removing 5 mm of the nerve rather than simply cutting prevents healing and regeneration of the nerve.
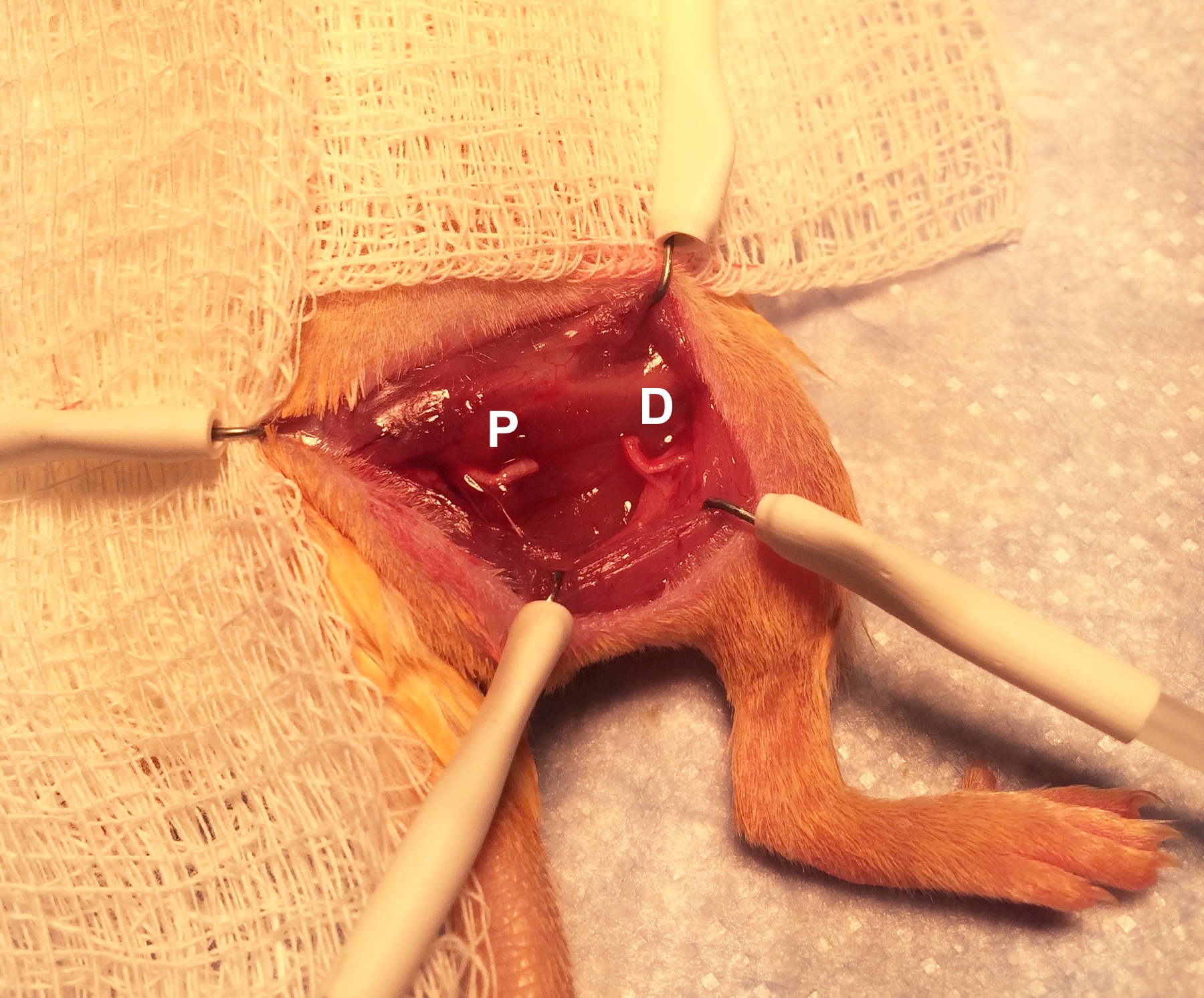
Figure 4. Division of the sciatic nerve. Removing 5 mm of the nerve prevents regeneration. - Cauterize the cut end of the proximal nerve with the hand-held cautery for 1 sec to further prevent regeneration. (In other words, cauterize the end of the nerve that is still attached to the spine, rather than the end attached to the distal limb.).
- If there is any bleeding, hold pressure with a gauze to obtain hemostasis. Close the skin incision with several interrupted 4-0 absorbable sutures (Figure 5).
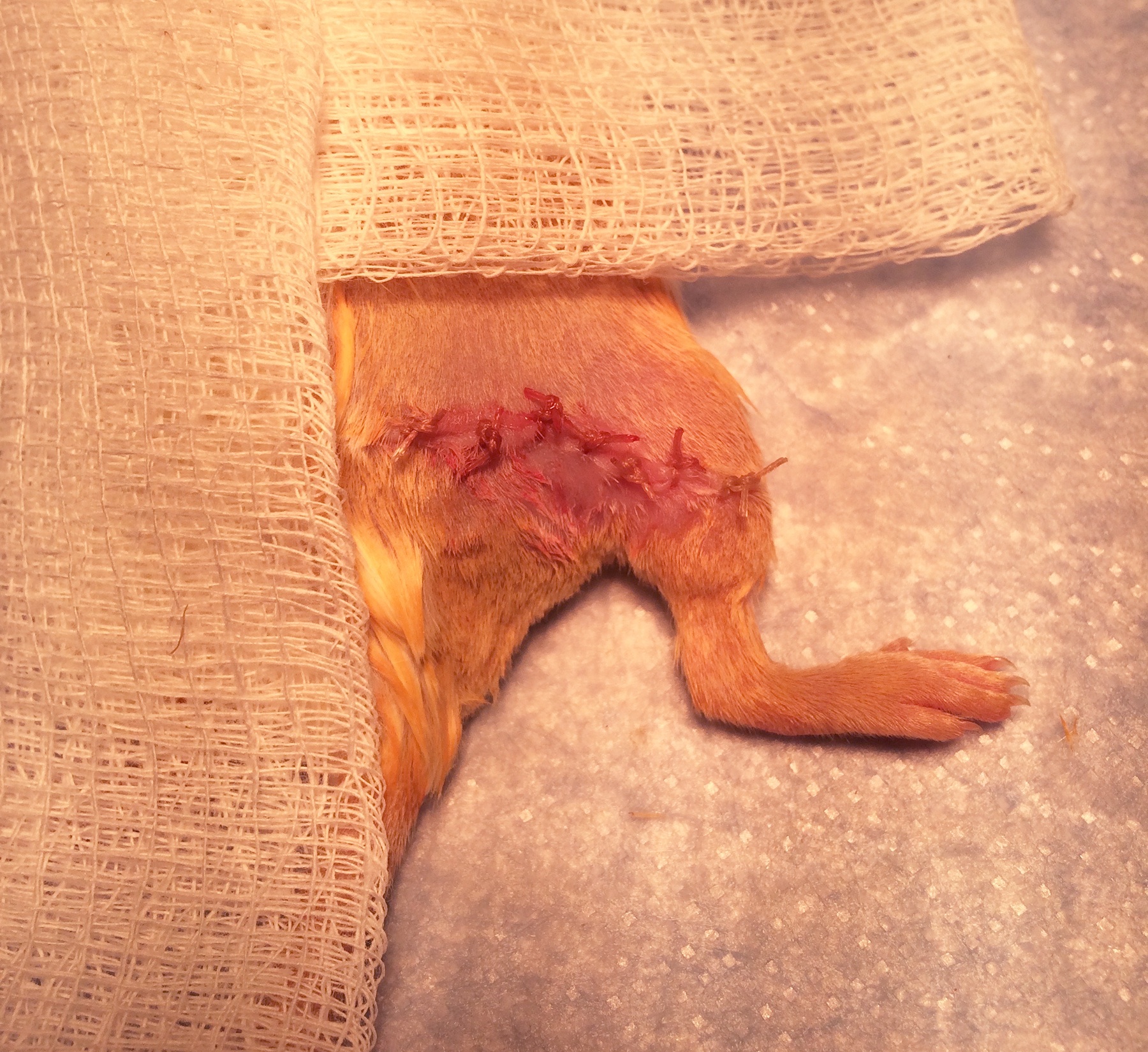
Figure 5. Closure of the posterior incision. Sutures should be spaced closely enough that no gaps remain in the incision.
- Place the mouse in prone position on a clean, absorbent surface on top of a regulated heating pad.
- Femoral nerve denervation
- Place the mouse in supine position on a clean, absorbent surface on top of a regulated heating pad.
- Shave the hair from the lower abdomen, groin, and entire thigh on the side to be operated on (Figure 6).
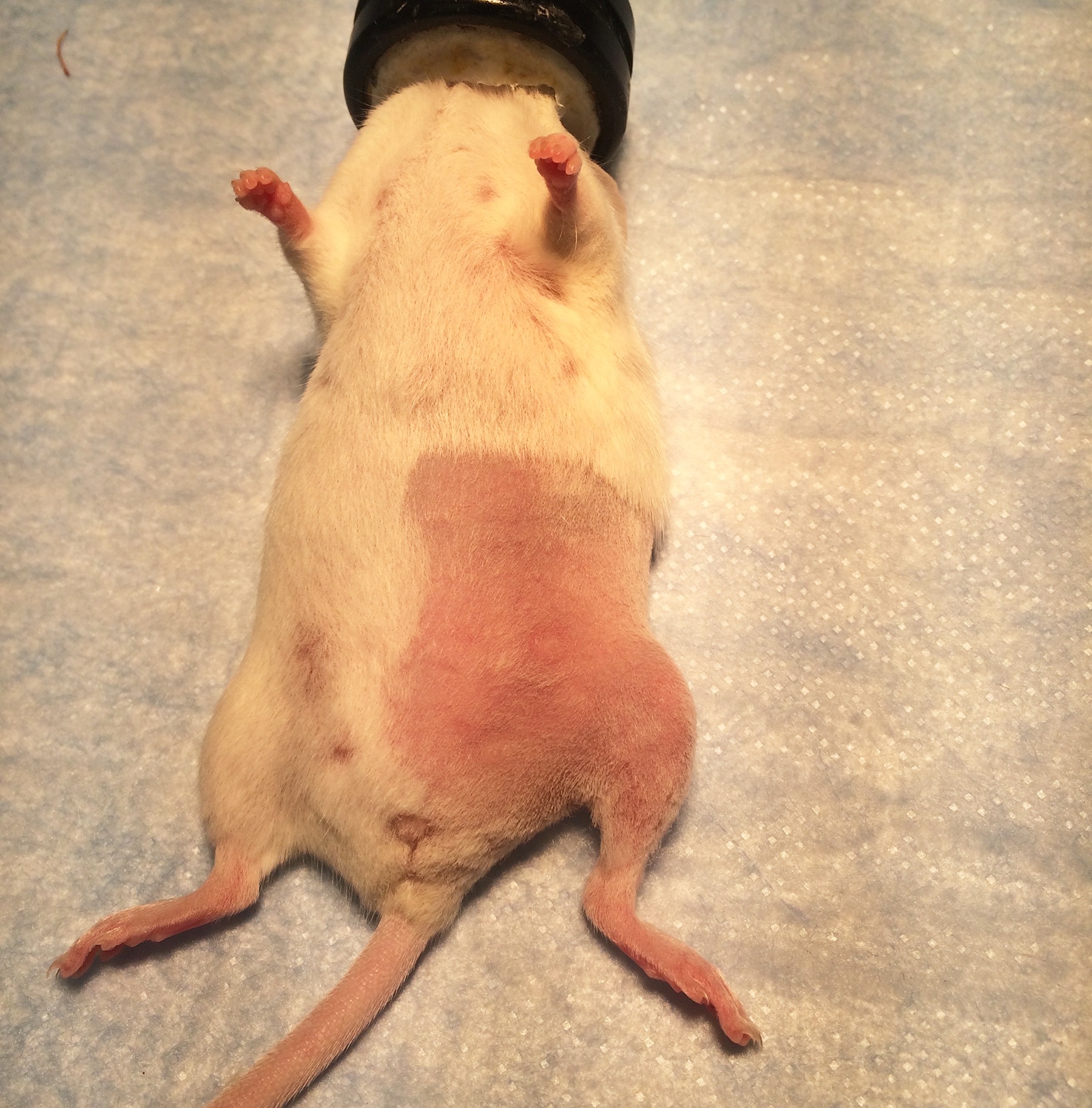
Figure 6. The anterior surgical site for femoral nerve access. Shave hair past the midline and halfway to the anterior limbs. - Aseptically prepare the shaved skin with povidone iodine solution and drape with sterile drapes. Make sure to maintain sterile technique during the entire surgery. Antibiotics are not needed as long as the operative field remains sterile.
- Make an incision parallel to the femur extending almost to the mouse’s midline. Using small sharp scissors, spread underneath the skin in order to loosen it from the underlying muscle.
- The femoral nerve, artery, and vein will be immediately visible running as a bundle parallel to the femur (Figure 7; arrowheads indicate the bundle.). Using the small sharp scissors with delicate movements, free the proximal part of the femoral nerve from the tissue surrounding it (Figure 8; arrowheads indicate the proximal part of the femoral nerve draped over the scissors.). Cut away a 5 mm segment of the nerve near the abdominal wall and use the hand-held cautery to cauterize for 1 sec the cut end of the proximal part of the nerve.
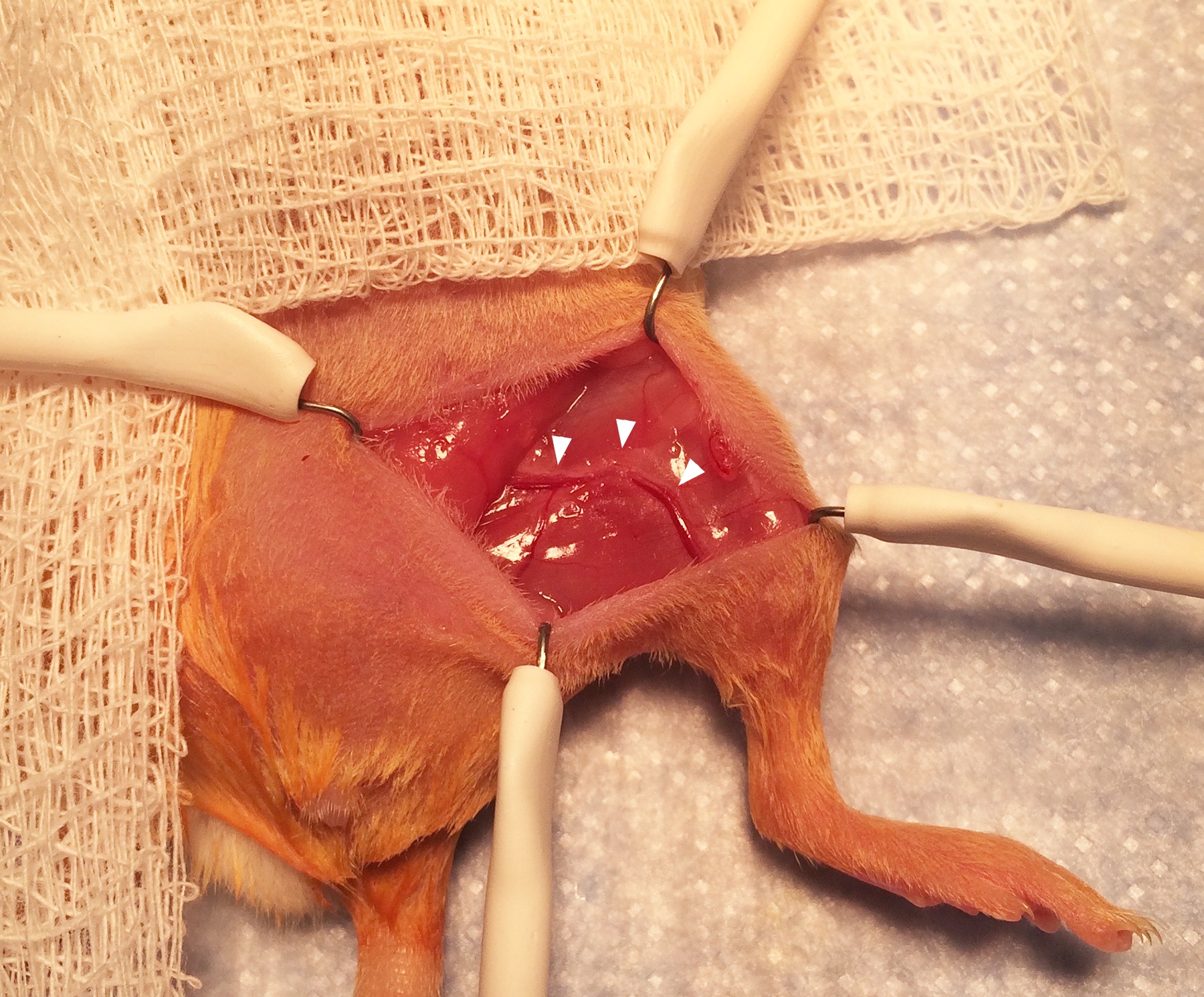
Figure 7. Exposure of the femoral nerve and artery. Separating the proximal femoral nerve from the artery and vein requires very delicate dissection using fine scissors.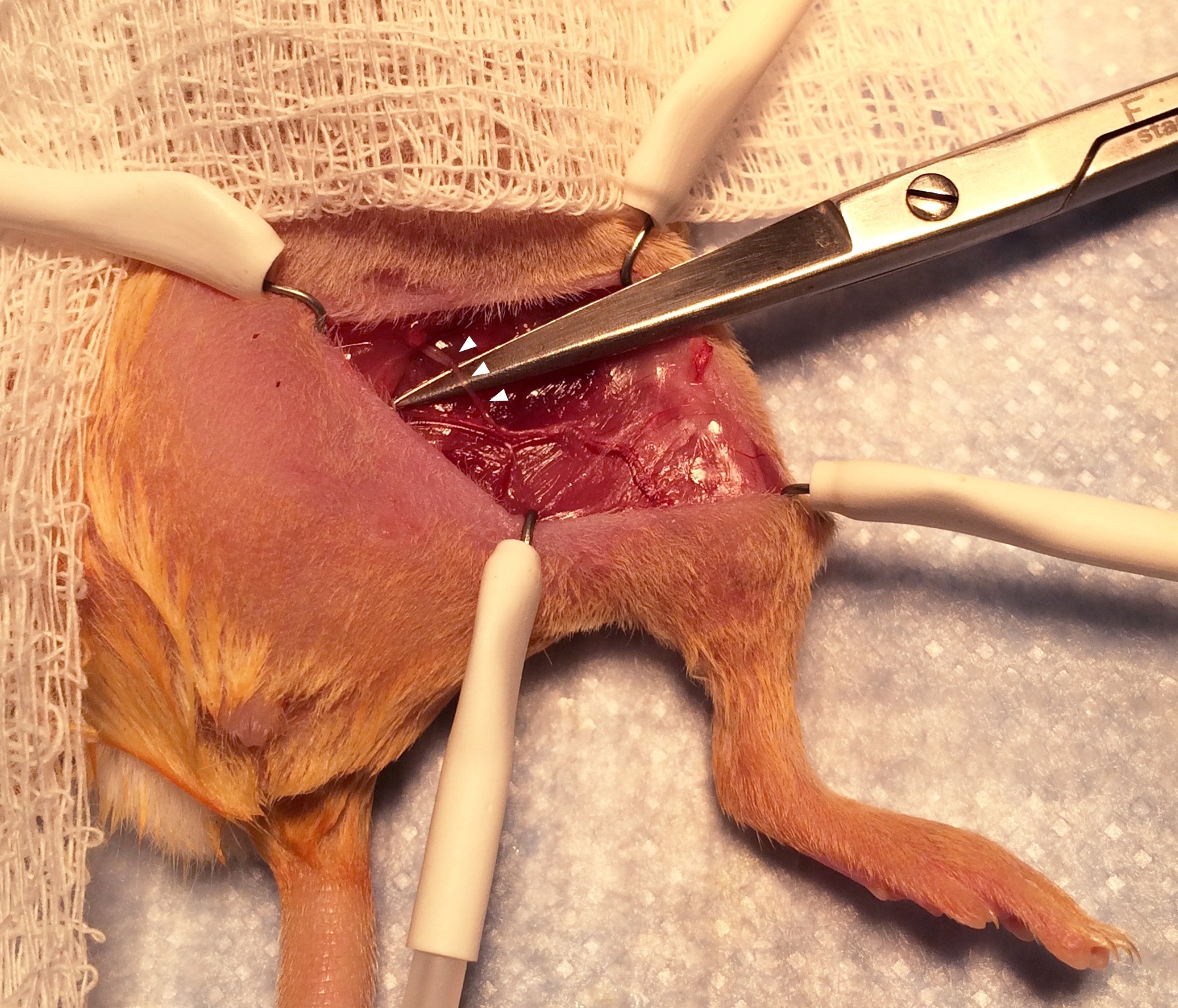
Figure 8. Exposure of the proximal femoral nerve. The portion of the nerve to be divided is seen draped over the scissors. Be careful to avoid shearing the femoral blood vessels. - Be careful not to disturb the femoral artery and vein. If bleeding from these vessels is accidentally caused, apply firm pressure with a gauze pad. If the bleeding cannot be stopped after 5 min of holding pressure, the animal should be euthanized immediately. Our typical method of euthanasia is to place the still-anesthetized mouse into a CO2 chamber until respirations cease, and to then perform cervical dislocation. However, it is best to consult with an institutional veterinarian before starting the procedure to determine the preferred method for euthanasia.
- Close the skin incision with several interrupted 4-0 absorbable sutures.
- Place the mouse in supine position on a clean, absorbent surface on top of a regulated heating pad.
- Ensure that adequate institutional animal care approvals are in place for the denervation procedure. Induce anesthesia with 2-3% inhaled isoflurane. Apply ophthalmic ointment to both eyes in order to prevent desiccation of the cornea during surgery.
- Post-procedure care monitoring
- After surgery, allow the mouse to recover in a paper-lined cage on top of a heating pad. Monitor the respiratory rate and pattern, response to stimuli, and ability to resume normal activity every 15 min until fully awake (Figure 9). Once the mouse is fully recovered from anesthesia and is ambulatory, place it into a clean home cage. Make sure to clean most of the iodine off of the skin and fur using warm water before the mouse is awake.
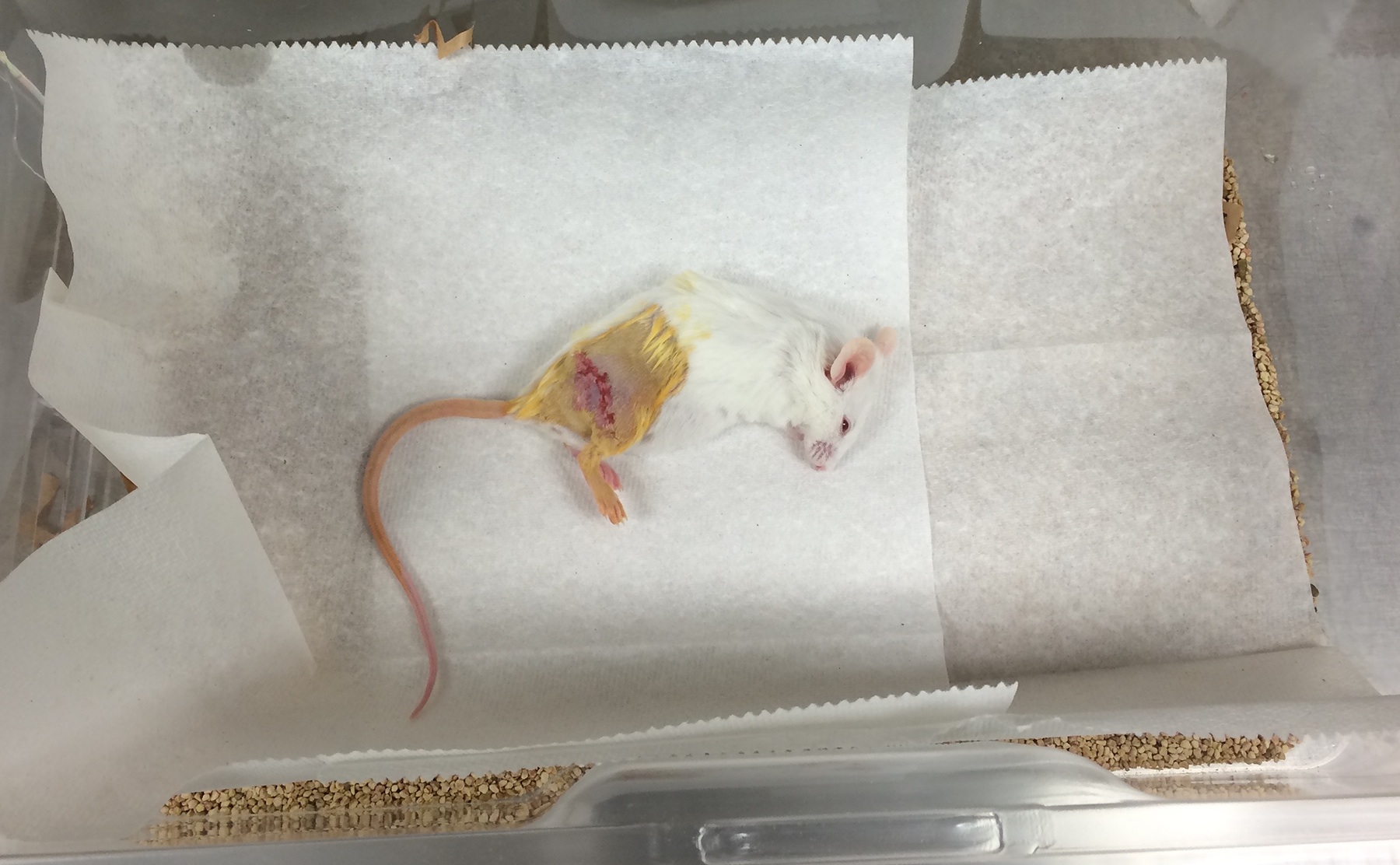
Figure 9. A mouse recovering from surgery. The mouse is placed onto clean paper towels to avoid aspiration of cage bedding. Monitor the mouse until it is able to ambulate. - After the inhaled anesthetic is stopped, the mouse’s respiratory rate is expected to return to the normal rate of 80-200 and it should begin responding to stimuli within 5 min and resuming normal activity within 10 min. A mouse whose respiratory rate stays consistently below 60 respirations per minute, who does not respond to stimuli after 5 min, or who does resume normal activity after 10 min may not recover and should be considered for euthanasia.
- After surgery, allow the mouse to recover in a paper-lined cage on top of a heating pad. Monitor the respiratory rate and pattern, response to stimuli, and ability to resume normal activity every 15 min until fully awake (Figure 9). Once the mouse is fully recovered from anesthesia and is ambulatory, place it into a clean home cage. Make sure to clean most of the iodine off of the skin and fur using warm water before the mouse is awake.
- Early euthanasia criteria
For one week after surgery, monitor the mice daily for their general appearance, activity level, weight loss, signs of infection, and development of a large fluid-filled cyst in the region. If these signs develop, consult with a veterinarian or euthanize the animal. - Procedure endpoint
Based on the requirements of the experiment, the mice are followed for varying amounts of time before being euthanized and the limb harvested. In our group we wait 8-12 weeks before harvesting the tissue.
Notes
This protocol completely removes any sciatic nerve input to the hind limb. However, femoral nerve branches innervate cutaneous tissues of the leg and foot, as well as ligaments, joints and blood vessels and may overlap with territories supplied by the sciatic nerve. We therefore wished to ensure that complete denervation on hind limb tissues took place, and that femoral nerve supply does not substitute sciatic nerve requirements (electrically, chemically, or functionally) after sciatic denervation. To accomplish this, surgical denervation of both sciatic and femoral nerves was performed, and two weeks prior to digit tip amputations to allow full nerve degeneration to take place. Complete nerve degeneration can be verified by monitoring beta 3 tubulin immunoreactivity on histological sections of denervated hind limbs. While ablation of sciatic innervation alone denervates the distal limb, femoral ablation was performed to ensure that no nerve regeneration from the proximal limb would occur during the regeneration time interval. Additionally, an incision was made in the ventral skin of the thigh and the femoral fascial sheath. Femoral denervation was achieved by cutting a 5 mm section of the femoral nerve, leaving the femoral artery intact. Sham surgeries were performed by exposing the nerves without cutting them.
Acknowledgments
Y.R. was supported by the Machiah Foundation Fellowship, the Siebel Stem Cell Institute and the Thomas and Stacey Siebel Foundation (1119368-104-GHBJI), the Human Frontier Science Program (HFSP) Long Term Fellowship, and partly by the HFSP Career Development Award (CDA00017/2016) and from the German Research Foundation (DFG, RI 2787/1-1 AOBJ: 628819). C.D.M. is supported by the American College of Surgeons Resident Research Scholarship.
References
- Kumar, A., Godwin, J. W., Gates, P. B., Garza-Garcia, A. A. and Brockes, J. P. (2007). Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318(5851): 772-777.
- Rinkevich, Y., Montoro, D. T., Muhonen, E., Walmsley, G. G., Lo, D., Hasegawa, M., Januszyk, M., Connolly, A. J., Weissman, I. L. and Longaker, M. T. (2014). Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc Natl Acad Sci U S A 111(27): 9846-9851.
- Singer, M. (1947). The nervous system and regeneration of the forelimb of adult Triturus; the relation between number of nerve fibers and surface area of amputation. J Exp Zool 104(2): 251-265.
- Singer, M. (1952). The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol 27(2): 169-200.
- Stover, S. L., Hale, A. M. and Buell, A. B. (1994). Skin complications other than pressure ulcers following spinal cord injury. Arch Phys Med Rehabil 75(9): 987-993.
- Stover, S. L., Omura, E. F. and Buell, A. B. (1994). Clinical skin thickening following spinal cord injury studied by histopathology. J Am Paraplegia Soc 17(2): 44-49.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Rinkevich, Y., Montoro, D. T., Muhonen, E., Lo, D., Hasegawa, M., Marshall, C. D., Walmsley, G. G., Connolly, A., Weissman, I. L. and Longaker, M. T. (2016). Denervation of Mouse Lower Hind Limb by Sciatic and Femoral Nerve Transection. Bio-protocol 6(13): e1865. DOI: 10.21769/BioProtoc.1865.
Category
Neuroscience > Neuroanatomy and circuitry > Sciatic nerve > Denervation
Neuroscience > Neuroanatomy and circuitry > Femoral nerve > Denervation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









