- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
T Cell Transfer Model of Colitis
Published: Vol 6, Iss 13, Jul 5, 2016 DOI: 10.21769/BioProtoc.1862 Views: 19426
Reviewed by: Ivan ZanoniAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2244 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC) is mainly caused by disordered immune regulation and dysregulated intestinal microbiota. Here we present the T cell transfer model which has extensively used in many studies to identify the regulatory T cell function in gut inflammation. Naïve T cells only or together with Treg cells isolated from different donors were transferred into immunodeficient Rag1-/- mice and the disease progression was assessed by the loss of body weight and the scoring analysis. This model provides a reliable work system for the study of gut inflammation.
Keywords: Induced colitisMaterials and Reagents
- 15 ml polypropylene conical tubes (Corning, catalog number: 430790 )
- 50 ml polypropylene conical tubes (Corning, catalog number: 430828 )
- LS columns (Miltenyi Biotec, catalog number: 130-042-401 )
- 6-8 weeks old C57BL/6J WT mice used for donors (males)
- 6-8 weeks old Immunodeficient RAG1−/− mice (males) (The Jackson Laboratory, Stock number: 00 2216 )
- 10 cm petri dish (Corning, catalog number: 430167 )
- 10 ml syringe (BD Biosciences, catalog number: 301604 )
- 27 gauge needle (BD Biosciences, catalog number: 305109 )
- 40 μm nylon cell strainer (Corning, Falcon®, catalog number: 352340 )
- 70 μm cell filter (Corning, Falcon®, catalog number: 352350 )
- Polystyrene round-bottom flow tubes (Corning, Falcon®, catalog number: 352054 )
- FBS (Thermo Fisher Scientific, GibcoTM, catalog number: 10100-147 )
- RPMI media 1640 (Thermo Fisher Scientific, GibcoTM, catalog number: 11875-093 )
- ACK lysing buffer for erythrocyte lysis (Thermo Fisher Scientific, GibcoTM, catalog number: A10492-1 )
- Mouse CD4 Negative Isolation Kit (Miltenyi Biotec, catalog number: 130-095-248 )
- Fluorescently labeled antibodies:
- anti-CD25-PE (BioLegend, catalog number: 102012 )
- anti-CD4-PE-Cy7 (BD Biosciences, catalog number: 563933 )
- anti-CD44-APC (BD Biosciences, catalog number: 559250 )
- anti-CD62L-FITC (BD Biosciences, catalog number: 553150 )
- 37% Formaldehyde (formalin) Solution (Avantor Performance Materials, J.T.Baker®, catalog number: 2016 )
- FACS buffer (see Recipes)
Equipment
- Plain glass microscope slides (Omano, catalog number: OMSK-50PL )
- Hemocytometer
- Light microscope
- Top-loading balance
- Centrifuge
- Flow cytometry (BD Biosciences, model: ARIA III )
Procedure
- Enrichment for CD4+ T cells using magnetic beads
- Euthanize donor WT mice.
- Harvesting spleen from mouse.
- Place the dissected organs in 10 cm petri dish with 10 ml PBS + 2% FBS. Use two pieces of sterile frosted glass (keep in -20 °C for 1 h in advance) slides to gently grind the tissues. For detail grinding procedure, please refer to http://www.bio-protocol.org/e834.
- Place 70 μm cell filter on top of a 50 ml tube and transfer fluid from dish to filter.
- Spin spleen suspension for 5 min at 4 °C, 300 x g (Note: This should be the centrifugation condition for all steps.). Discard supernatant by aspiration with care not to disturb the pellets.
- Add 4 ml ACK lysis buffer and incubate at room temperature for 3 min. Add another 10 ml PBS + 2% FBS.
- Place 40 μm cell filter on top of a 50 ml tube and transfer fluid from 50 ml tube to filter. Spin for 5 min and discard supernatant by pouring off.
- Wash cells 2 times with 10 ml PBS + 2% FBS. Resuspend cells with PBS + 2% FBS buffer. Take an aliquot to count cells. Add 10 μl of cell suspension to 90 μl of Trypan blue solution (1:10 dilution) and count cells using a hemocytometer.
- Enrich CD4+ T cells by using the CD4+ T Cell Isolation Kit from Miltenyi Biotec.
- Following the negative selection protocol, pellet cells by centrifugation at 300 x g at 4 °C for 10 min.
- Discard most of the supernatant by aspiration; resuspend the cell pellet in 1 ml PBS + 2% FBS buffer by carefully pipetting up and down several times. Take an aliquot to count cells. Add 10 μl of cell suspension to 90 μl of Trypan blue solution (1:10 dilution) and count cells using a hemocytometer.
- Euthanize donor WT mice.
- Cell surface antigen staining for cell sorting
- We transfer appropriate number of cells into FACS tube and spin at 300-400 x g for 5 min.
- Prepare antibody cocktail containing anti-CD4-PE-Cy7, anti-CD25-PE, anti-CD44-APC, anti-CD62L-FITC antibodies diluted in 1x PBS with 2% FBS to optimal concentration (1:100). For every million cells, we usually add 100 μl 1x PBS with 2% FBS buffer with diluted antibody for incubation. According to the ratio and cells to be stained, prepare an antibody/PBS master mix.
- Resuspend the cells in 1x PBS with 2% FBS to obtain concentration of 25-50 x 106 cells/ml. The concentration of cells is dependent on the specific cell sorter to be used. Add the above mixture to cell pellet. Vortex to mix well. Stain in 4 °C for 15 min.
- Wash cells twice by filling tube with 1x PBS + 2% FBS. Spin cells down at 300 x g for 5 min at 4 °C. Pour off liquid.
- If debris or clumps are visible it is necessary to pass cells through a 40 μm cell strainer prior to cell sorting
- Protected cells from light and placed on ice prior to sorting.
- Sort naive T cells (CD4+CD25-CD44loCD62Lhi) and Treg populations (CD4+CD25hi) into RPMI media 1640 with 2% FBS.
- We transfer appropriate number of cells into FACS tube and spin at 300-400 x g for 5 min.
- Injection of RAG1−/− mice with flow-purified naïve T cells and Treg cells
- After sorting, fill the collection tubes containing sorted cells with cold 1x PBS to collect cells in the walls and pellet by centrifugation as described above. Always keep cells on ice to avoid clump in each step.
- Wash cells 2 times with cold 1x PBS. This step is intended to deplete trace FBS. Resuspend cells very gently and prepare a single-cell suspension with PBS only and placed the cells on ice prior to injection. Take an aliquot to count cells. Add 10 μl of cell suspension to 90 μl of Trypan blue solution (1:10 dilution) and take 10 μl diluted cell suspension out to count cells using a hemocytometer.
- After counting, dilute cells with cold 1x PBS and adjust into a final cell concentration of 2 x 106/ml. Keep injection tubes on ice prior to injection.
- Mix cells for different groups. For every mouse, either 4 x 105 naïve T cells alone (200 μl) or 4 x 105 naïve T (200 μl) together with 2 x 105 Treg cells (100 μl) are mixed.
- Weigh recipient mice using a top-loading balance and record the weight. Prior to the injection of the mice gently invert the tube(s) to mix cells.
- Restrain the mouse use a tail access mouse restrainer. For detail restrainer picture, please refer to: http://www.stoeltingco.com/neuroscience/misc/restrainers.html. Rotate the tail slightly to visualize vein. Very slowly draw the cells into 1-ml syringe with the 27 gauge needle attached. Insert needle into the vein at a slight angle and inject all cells in.
- After sorting, fill the collection tubes containing sorted cells with cold 1x PBS to collect cells in the walls and pellet by centrifugation as described above. Always keep cells on ice to avoid clump in each step.
- Monitoring disease progression and assessment of colitis
Monitor disease progression by weighing each mouse twice per week using a top-loading balance. The mice get typically slow but progressive weight loss in the following 5-7 wk, accompanied by appearance of loose stools and diarrhea as well as a “hunched-over” appearance.
Note: Some mice do not develop significant loose stools and diarrhea. However, these mice may still develop significant intestinal inflammation, which will be visible upon H&E staining.- When mice lose about 15-20% of their original body weight or at about 40 d post injection, the animals should be euthanized and assessed for evidence of colitis.
- Carefully excise the entire colon starting from the anus and cutting it free of the mesentery from below the cecum to just above the anal opening. Be careful not to stretch of the colon.
- Place the colon on a plastic board, measure and record its length. Then clean it of fecal material by injecting PBS. Weigh the colonic tissue
- Colonic length and weight may be recorded to calculate length-to-weight ratio, which has been shown to correlate well with blinded histopathological scores.
- For histological processing and blinded histopathological scoring, a small (0.5 cm) piece from proximal and distal portions of the colon is obtained. These pieces of tissue are then placed in 10% PBS-formalin and allowed to fix for at least 24 h at room temperature prior to paraffin embedding.
- Embed fixed tissue into paraffin blocks. Section the paraffin-embedded samples produce 4-μm sections. Mount three sections on a microscopic glass slide, stain the sections with hematoxylin and eosin.
- Perform blinded histopathological analysis using the following scoring criteria:
- Degree of inflammation in lamina propria (score 0-3)
- Goblet cell loss (score 0-2)
- Abnormal crypts (score 0-3)
- Presence of crypt abscesses (score 0-1)
- Mucosal erosion and ulceration (score 0-3)
- Submucosal spread to transmural involvement (score 0-3)
- Number of neutrophils counted at 40x magnification (score 0-4)
- Degree of inflammation in lamina propria (score 0-3)
- When mice lose about 15-20% of their original body weight or at about 40 d post injection, the animals should be euthanized and assessed for evidence of colitis.
Representative data
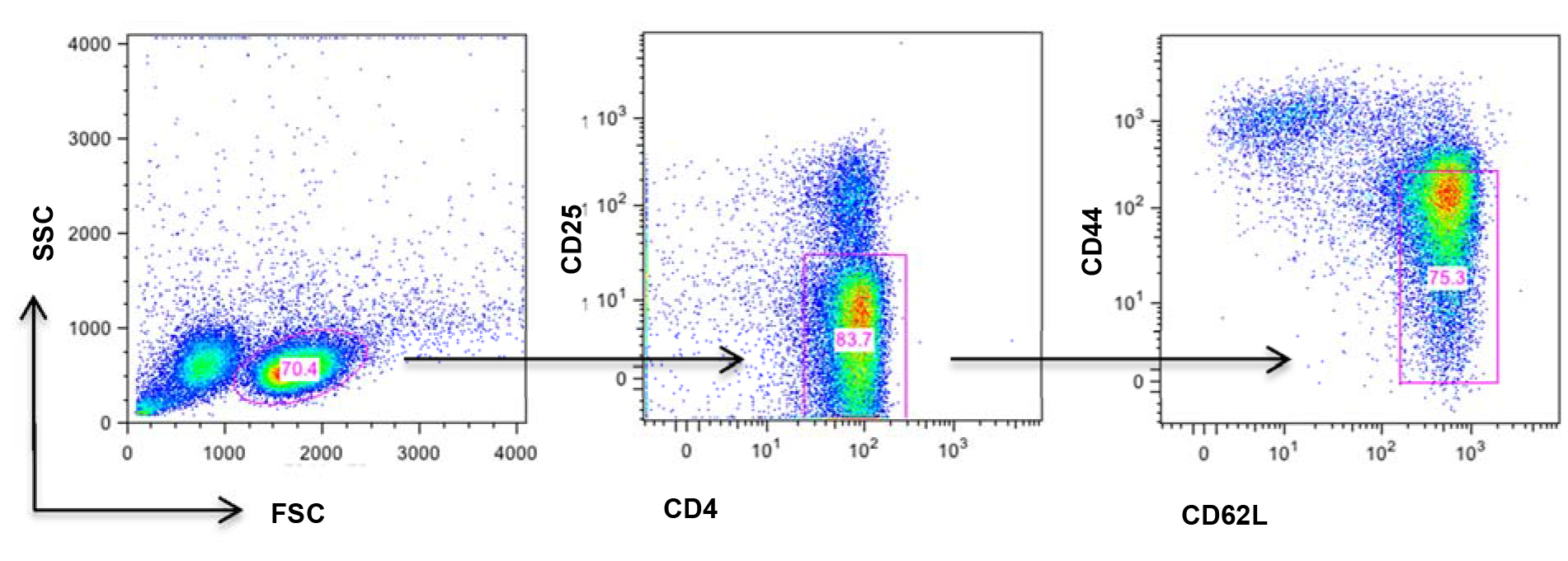
Figure 1. Schematic illustration of naïve T cell markers. Splenic cells were resuspended and stained with antibody cocktail containing anti-CD4-PE-Cy7, anti-CD25-PE, anti-CD44-APC, anti-CD62L-FITC antibodies for in 4 °C for 15 min. Naive T cells (CD4+CD25-CD44loCD62Lhi) were gated as above.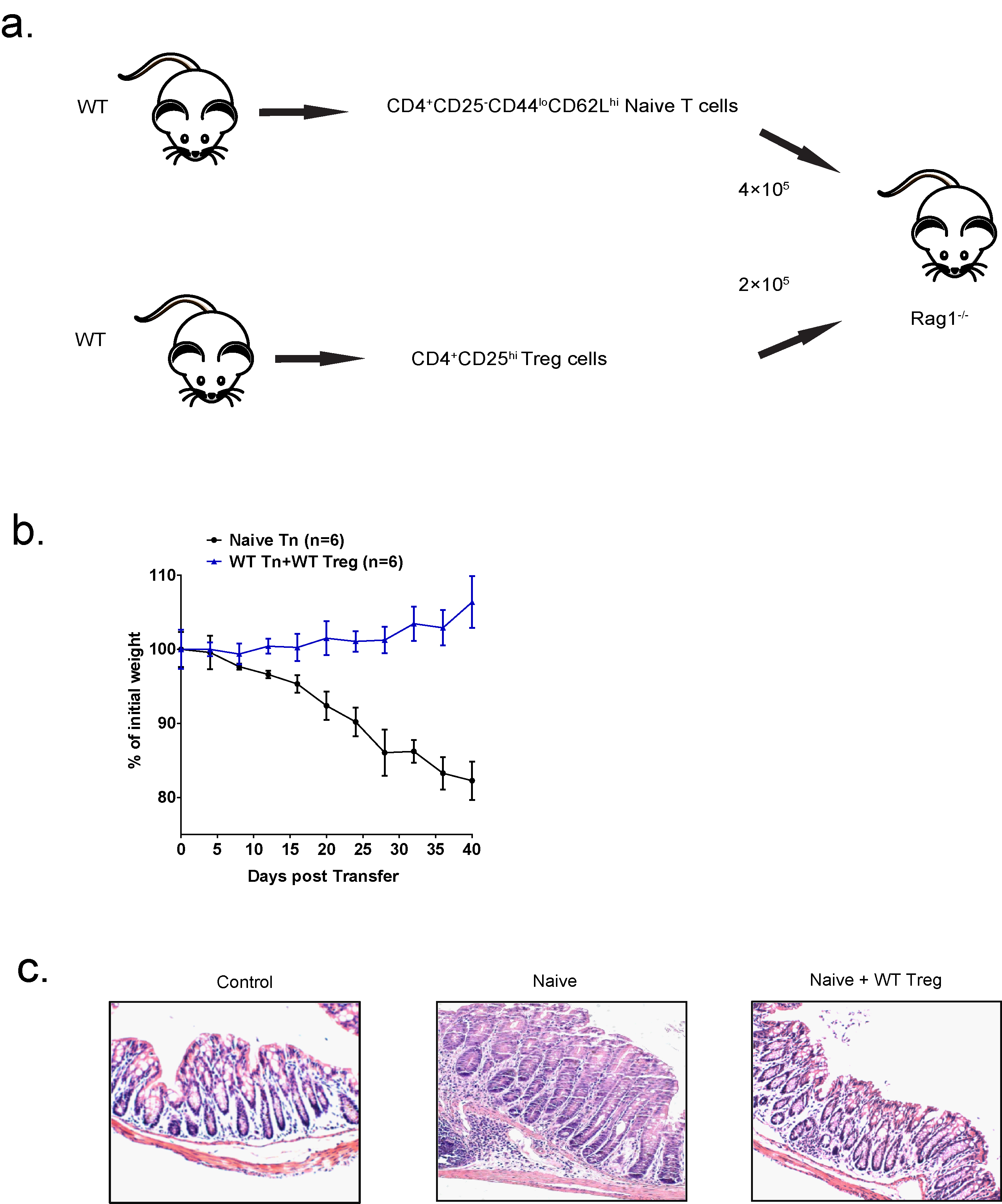
Figure 2. Schematic illustration of how T cell transfer model of colitis was investigated. Each Rag1–/– recipient mouse was transferred with 4 x 105 CD4+CD25-CD44loCD62Lhi naive T cells isolated from wild-type mice, and co-transferred with 2 x 105 CD4+CD25hi Treg cells isolated from WT (b) The weight loss of the mice in (a) was monitored every four days for 40 days from the day after injection. n = 6 mice for each group. (c) Representative H&E staining of colon sections from untreated WT mice (control), Rag1–/– mice transferred with WT naive T cells (naive), Rag1–/– mice co-transferred with WT naive T cells and WT Treg cells (naive + WT Treg (original magnification, 20x).
Notes
- Transfer results in no colitis development. Possible causes:
- Low naive T cell numbers transferred.
Cell viability is another critical factor. Make sure the cells are always maintained on ice. It is important to assess the cell viability after the sorting is completed and only >90% viability is acceptable for transfer. - It is important to remember that this model depends on many additional factors apart from the viability of naive T cells, sufficient naive T cells, and good animal procedures. These include the cleanliness of the animal house and the intact intestinal flora in recipient mice.
Recipes
- FACS buffer
2% FBS in PBS, keep cold for staining.
Acknowledgments
This protocol was modified from our previous work by Pan et al. (2015). This work is supported by grants from 973 program (2014CB541902, 2014CB541901), National Natural Science Foundation of China (81230072, 81025016, 31370880), the Key Research Program of the Chinese Academy of Sciences (KSZD-EW-Z-003-3), as well as Chinese Ministry of Health (201202008) and the Program of the Shanghai Commission of Science and Technology (12431900703, 12JC1406000, 12ZR1435900).
References
- Franke, A., McGovern, D. P., Barrett, J. C., Wang, K., Radford-Smith, G. L., Ahmad, T., Lees, C. W., Balschun, T., Lee, J., Roberts, R., Anderson, C. A., Bis, J. C., Bumpstead, S., Ellinghaus, D., Festen, E. M., Georges, M., Green, T., Haritunians, T., Jostins, L., Latiano, A., Mathew, C. G., Montgomery, G. W., Prescott, N. J., Raychaudhuri, S., Rotter, J. I., Schumm, P., Sharma, Y., Simms, L. A., Taylor, K. D., Whiteman, D., Wijmenga, C., Baldassano, R. N., Barclay, M., Bayless, T. M., Brand, S., Buning, C., Cohen, A., Colombel, J. F., Cottone, M., Stronati, L., Denson, T., De Vos, M., D'Inca, R., Dubinsky, M., Edwards, C., Florin, T., Franchimont, D., Gearry, R., Glas, J., Van Gossum, A., Guthery, S. L., Halfvarson, J., Verspaget, H. W., Hugot, J. P., Karban, A., Laukens, D., Lawrance, I., Lemann, M., Levine, A., Libioulle, C., Louis, E., Mowat, C., Newman, W., Panes, J., Phillips, A., Proctor, D. D., Regueiro, M., Russell, R., Rutgeerts, P., Sanderson, J., Sans, M., Seibold, F., Steinhart, A. H., Stokkers, P. C., Torkvist, L., Kullak-Ublick, G., Wilson, D., Walters, T., Targan, S. R., Brant, S. R., Rioux, J. D., D'Amato, M., Weersma, R. K., Kugathasan, S., Griffiths, A. M., Mansfield, J. C., Vermeire, S., Duerr, R. H., Silverberg, M. S., Satsangi, J., Schreiber, S., Cho, J. H., Annese, V., Hakonarson, H., Daly, M. J. and Parkes, M. (2010). Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42(12): 1118-1125.
- Pan, W., Zhu, S., Dai, D., Liu, Z., Li, D., Li, B., Gagliani, N., Zheng, Y., Tang, Y., Weirauch, M. T., Chen, X., Zhu, W., Wang, Y., Chen, B., Qian, Y., Chen, Y., Fang, J., Herbst, R., Richman, L., Jallal, B., Harley, J. B., Flavell, R. A., Yao, Y. and Shen, N. (2015). MiR-125a targets effector programs to stabilize Treg-mediated immune homeostasis. Nat Commun 6: 7096.
- Strober, W., Fuss, I. J. and Blumberg, R. S. (2002). The immunology of mucosal models of inflammation. Annu Rev Immunol 20: 495-549.
- Strober, W., Fuss, I. and Mannon, P. (2007). The fundamental basis of inflammatory bowel disease. J Clin Invest 117(3): 514-521.
- Xavier, R. J. and Podolsky, D. K. (2007). Unravelling the pathogenesis of inflammatory bowel disease. Nature 448(7152): 427-434.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Pan, W., Zhu, S., Dai, D., Tang, Y., Yao, Y. and Shen, N. (2016). T Cell Transfer Model of Colitis. Bio-protocol 6(13): e1862. DOI: 10.21769/BioProtoc.1862.
Category
Cell Biology > Cell isolation and culture > Cell isolation
Cell Biology > Cell imaging > Fixed-tissue imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










