- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Aspergillus terreus Infection of Fruits and Terrein Quantification by HPLC Analysis
Published: Vol 6, Iss 12, Jun 20, 2016 DOI: 10.21769/BioProtoc.1845 Views: 8833
Reviewed by: Arsalan DaudiKanika GeraTatsuki Kunoh

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2645 Views

In Vitro Screening of Microbial Extracts Against the Oomycetes Phytophthora capsici and Pythium ultimum
Mónica Trigal Martínez [...] María Ángeles Vinuesa Navarro
Sep 20, 2025 1265 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1582 Views
Abstract
The opportunistic fungal human and plant pathogen Aspergillus terreus (A. terreus) can be isolated from sea water, soil or decaying organic matter such as rotting leaves and fruits. While growing on fruits A. terreus produces secondary metabolites such as terrein, which may ease its penetration into plant tissues. In addition, biological activities of terrein may support competition against other microorganisms. In summary, terrein is a small polyketide that reduces germination of seedlings, induces lesions on fruit surfaces but also shows moderate antifungal activity. With this manuscript we provide a fruit infection protocol with Aspergillus terreus with subsequent determination of terrein production rates on infected fruits using an HPLC-based quantification approach.
Keywords: Aspergillus terreusMaterials and Reagents
- Petri dish with three cams (SARSTEDT AG & Co, catalog number: 82.1473 )
- T-shaped plastic spreaders (VWR International, catalog number: 30002-110 )
- 40 μm cell strainer (Corning, catalog number: 352340 )
- 50 ml screw cap tubes (Sigma-Aldrich, catalog number: T2318 )
- Glass beaker (1,000 ml)
- Scalpel, sterile (VWR International, catalog number: 95039-116 )
- 2 ml HPLC vials (VWR International, catalog number: 66020-950 )
- Plastic syringes with syringe filters (nylon, 0.45 μm) (Sigma-Aldrich, catalog number: Z260428 )
- Fluted cellulose filter MN 1672 (MACHEREY-NAGEL GmbH & Co. KG, catalog number: 57 20 11 )
- Aspergillus terreus conidia
- Strain SBUG844 (www.leibniz-hki.de/en/jena-microbial-resource-collection.html) [Jena Microbial Research Collection(JMRC)] or
- Strain FGSC A1156 (www.fgsc.net) [Fungal Genetics Stock Center(FGSC)]
- Organic fruits (preferable bananas, apples or peaches)
- KLEENEX® C-fold towels, Kimberly-Clark Professional® (cotton tissue) (VWR International, catalog number: 10815-990 )
- Deionised water, sterile
- Ethanol (Carl Roth GmbH + Co. KG, catalog number: 5054.4 )
- Ethyl acetate, acetic ester (Carl Roth GmbH + Co. KG, catalog number: 7336.1 )
- HPLC grade methanol (Carl Roth GmbH + Co. KG, catalog number: P717.1 )
- Terrein standard (Sigma-Aldrich, catalog number: T5705 )
- Formic acid (Sigma-Aldrich, catalog number: 56302 )
- D-Glucose (Carl Roth GmbH + Co. KG, catalog number: 6887.1 )
- NaOH (Carl Roth GmbH + Co. KG, catalog number: 6771.1 )
- Agar (Carl Roth GmbH + Co. KG, catalog number: 2266.1 )
- NaNO3 (Carl Roth GmbH + Co. KG, catalog number: A136.1 )
- KCl (Carl Roth GmbH + Co. KG, catalog number: 6781.1 )
- MgSO4·7H2O (Carl Roth GmbH + Co. KG, catalog number: P027.1 )
- KH2PO4 (Carl Roth GmbH + Co. KG, catalog number: 3904.1 )
- ZnSO4·7H2O (Carl Roth GmbH + Co. KG, catalog number: K301.1 )
- H3BO3 (Carl Roth GmbH + Co. KG, catalog number: 6943.1 )
- MnCl2·4H2O (Carl Roth GmbH + Co. KG, catalog number: T881.1 )
- FeSO4·7H2O (Carl Roth GmbH + Co. KG, catalog number: P015.1 )
- CoCl2·6H2O (Carl Roth GmbH + Co. KG, catalog number: T889.1 )
- CuSO4·5H2O (Carl Roth GmbH + Co. KG, catalog number: P024.1 )
- (NH4)6Mo7O24·4H2O (Sigma-Aldrich, catalog number: 09880-100G )
- Disodium ethylene diamine tetraacetate (Na2EDTA) (Sigma-Aldrich, catalog number: E9884-100G )
- KOH (Carl Roth GmbH + Co. KG, catalog number: 6751.1 )
- Aspergillus minimal medium (AMM) plates (see Recipes)
- 20x salt stock solution (see Recipes)
- 1,000x Hutner’s trace elements (see Recipes)
- HPLC solvent A (see Recipes)
- HPLC solvent B (see Recipes)
Equipment
- Porcelain pestles (VWR International, catalog number: 470149-080 )
- Counting cell chamber (Thoma chamber) (VWR International, catalog number: 101765-022 )
- Accuracy balance
- 500 ml round bottom flask (VWR International, catalog number: BOHLA158-09 )
- Magnetic stirrer
- Magnetic stirring bar (VWR International, catalog number: 58948-954 )
- Drying cabinet
- Rotary evaporator (e.g.,Carl Roth GmbH + Co. KG, catalog number: PE53.1 )
- High-performance liquid chromatography (HPLC) system equipped with a diode array detector (e.g., Agilent 1260 modular HPLC system) with bifunctional-phenylpropyl-modified C18 column (e.g., Sphinx RP, 4.0 x 3 x 250 mm; 5 mm) (MACHEREY-NAGEL GmbH & Co. KG, catalog number:760808.46) with a binary solvent system (solvents and running parameters see below)
Procedure
- Streak Aspergillus terreus conidia from a frozen glycerol stock (60%) onto Aspergillus Minimal Medium (AMM) plate (9.2 cm diameter, 25 ml medium solidified with 2% agar) and cultivate for 4 days at 37 °C in the dark (Figure 1A).
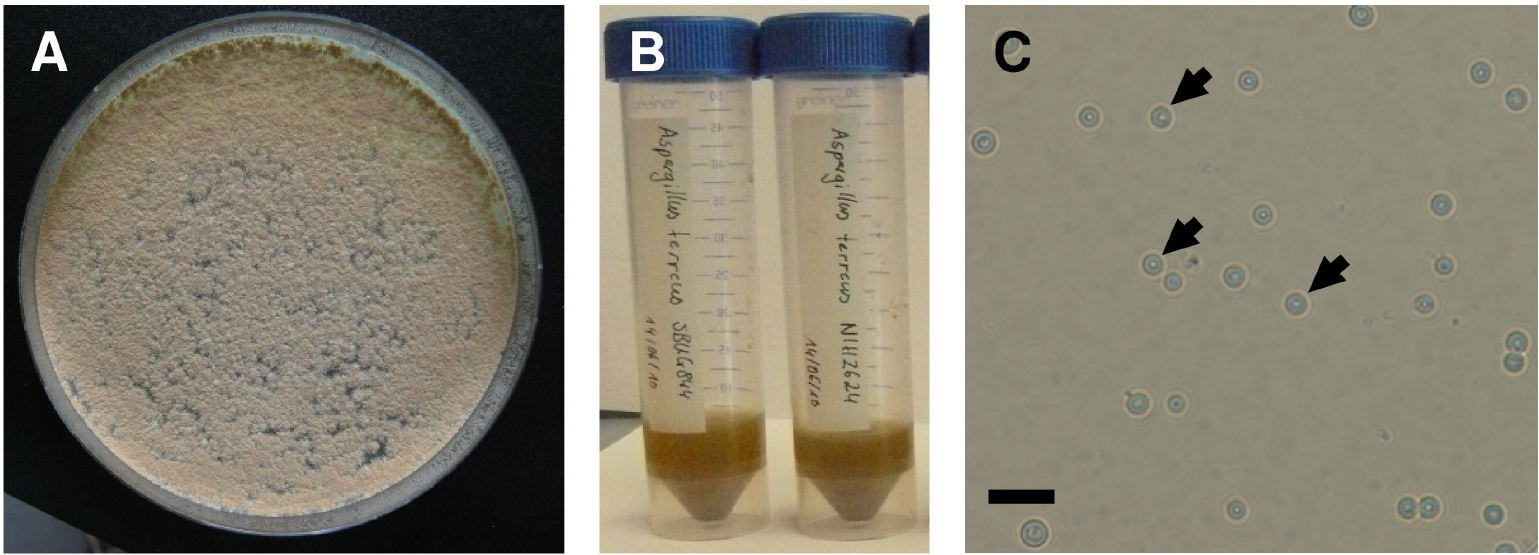
Figure 1. Preparation of Aspergillus terreus conidia suspensions. A. AMM agar plate with Aspergillus terreus grown for 4 d at 37 °C. Note the cinnamon coloured conidia on top of the colonies. B. Aqueous suspension of conidia. C. Microscopic image of Aspergillus terreus conidia (arrows). Note the round shape of the conidia and an average size of 2.5-3.0 μm. Scale bar = 10 μm. - Collect the conidia with a spreader by adding 10 ml distilled sterile water. Filter conidia over a 45 μm cell strainer to remove any clumps and hyphal fragments and collect in a 50 ml screw cap tube (Figure 1B).
- Centrifuge (10 min at 2,600 x g), wash the cells at least once with 10 ml distilled sterile water with subsequent second centrifugation (10 min, 2,600 x g) and suspend conidia in 5 ml distilled sterile water.
- Estimate the conidia concentration in suspension using a counting chamber (Thoma or Neubauer chamber) (Figure 1C). If necessary, dilute the crude suspension by factor 10 or 100 in sterile water prior to counting.
- Slightly wipe the surface of the fruits with 70% ethanol. Subsequently, wash with distilled water and dry the surface with a soft cotton tissue (Figure 2A).
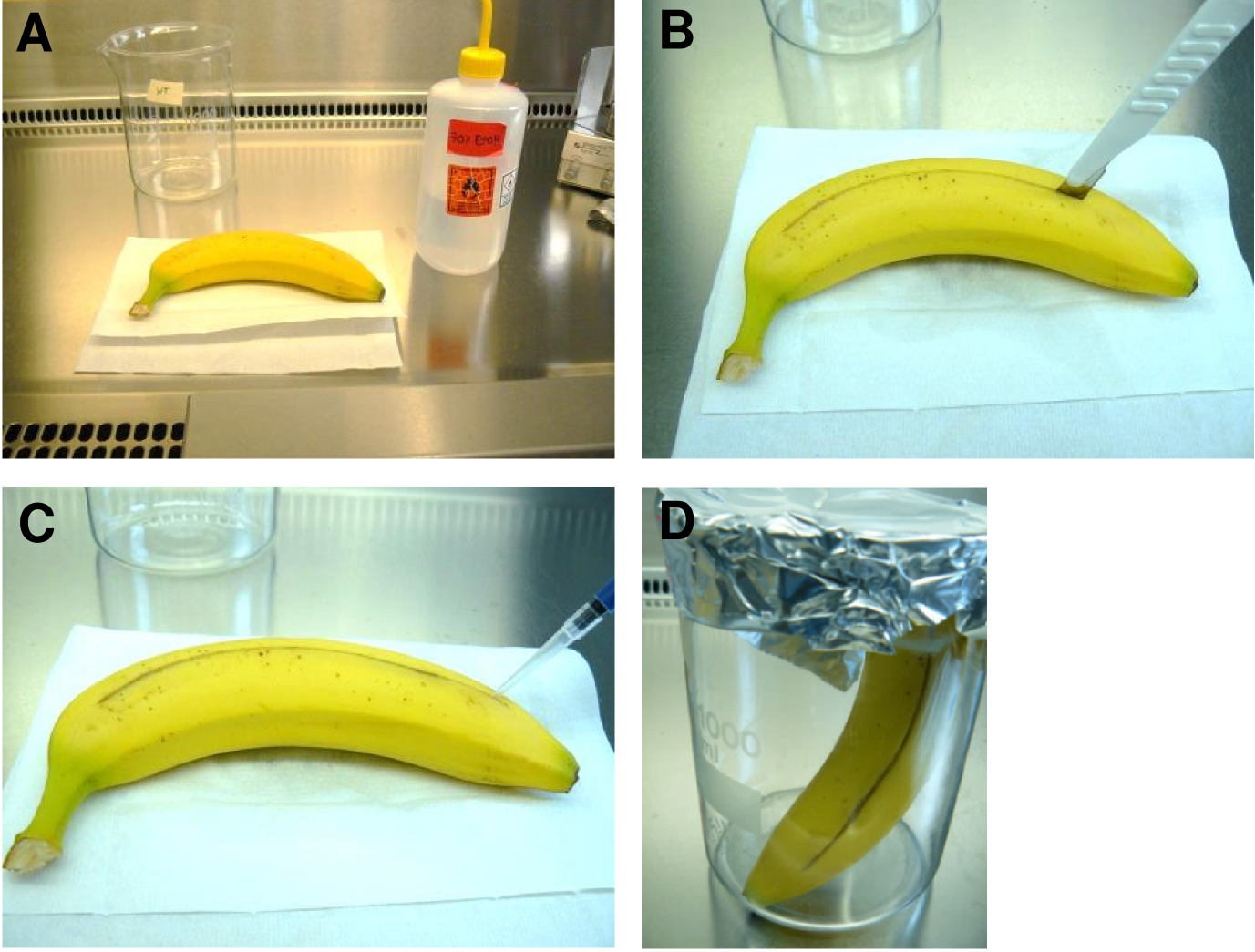
Figure 2. Preparation of fruits for fungal infection. A. Surface disinfection with 70% ethanol. B. Longitudinal sectioning with a sterile scalpel. C. Infection with conidia suspension in the longitudinal groove. D. Incubation in a sterile glass beaker (pre-weighed) for 5 to 7 days at room temperature. - Cut the fruit longitudinally with a sterile scalpel (length around 10 cm; depth around 1.5 cm) (Figure 2B).
- Inoculate with 200 μl of a conidia suspension containing 2 x 108 conidia/ml. Disperse the volume evenly over the complete length of the groove (Figure 2C). For negative control use 200 μl of sterile water. Fruits used for infection and for the control must be taken from the same batch! At least three technical replicates (three fruits, each from the same batch or parental plant) and five biological (five different batches) replicates should be carried out.
- Weigh a sterile 1,000 ml glass beaker including a covering aluminium foil.
- Put the fruit into the sterile 1,000 ml beaker glass and cover it with aluminium foil (Figure 1D).
- Incubate the fruit for 5 to 7 days at room temperature (Figure 3).
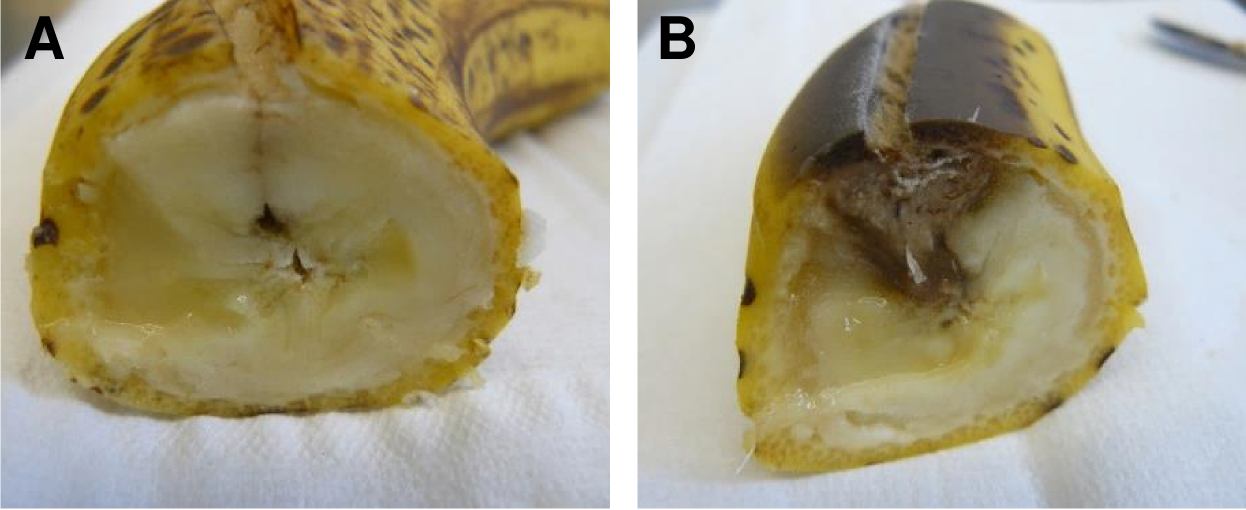
Figure 3. Cross-section of bananas after 7 days of incubation. A. Control (mock) infected banana. B. Banana infected with 200 μl of A. terreus conidia suspension (2 x 108 conidia/ml). - Cut the fruit into pieces of approximately 2 x 2 cm size and smash the fruit in the glass beaker using a pestle.
- Add a magnetic stir bar and pour 150 ml ethyl acetate into the beaker.
- Extract the fruits while stirring on a magnetic stirrer at 150 rpm for 15 min.
- Decant the supernatant into a 500 ml round bottom flask and repeat the extraction procedure. Pool both extracts.
- For dry weight determination of bananas evaporate the fruit debris in the glass beaker under a fume hood. Subsequently dry it at 37-50 °C. Weigh the glass beaker including its cover and calculate the dry weight by subtracting the weight of the empty glass beaker as previously determined.
- Evaporate the ethyl acetate from the fruit extractions to dryness using a rotary evaporator.
- Solve the residue in 2 ml HPLC grade methanol. Dilute the extract in a ratio of 1:10, 1:20 and 1:50 in methanol. Filter the dilutions over a 0.45 μm nylon filter into a HPLC vial.
- Run a calibration curve by applying 10 μl serial dilutions of the terrein standard (2.5 to 500 μg/ml in methanol) to a bifunctional C18 column (e.g., Macherey-Nagel Sphinx RP, 4.0 x 3 x 250 mm; 5 mm) in a modular HPLC system equipped with DAD (e.g., Agilent 1260 modular HPLC system). The following gradient (solution A = water + 0.1% formic acid, solution B = methanol) with a flow rate of 0.8 ml/min should be applied: 0.5 min = 10% B, 0.5-20 min = 10%-70% B, 20-25 min = 70%-100% B, 25-28 min = 100% B, and 28-33 min = 100%-10% B. Terrein is detected at a wavelength of 254 nm.
- Generate a calibration curve from peak integrations at 254 nm.
- Apply 10 μl aliquots of the diluted fruit extracts to the column.
- Use the calibration curve to calculate the amount of terrein (μg) by peak integration from the UV profile. Note that the total volume of terrein extract in methanol was 2 ml.
- Determine the total terrein content as terrein (μg)/dry-weight of fruit (g).
Notes
- If the supernatant after the ethyl acetate extraction is not clear (contaminating fruit debris) the extract can be optionally filtered over a fluted cellulose filter.
- Typical terrein yields range from 10 to 450 μg terrein per (g) of fruit dry weight depending on the type of fruits and cultivation time. To increase the accuracy of measurements on a given type of fruit, at least 15 replicates (including technical and biological replicates) should be performed.
- Organic fruits should be used to avoid contaminating fungicides or pesticides, which could impede with fungal growth and terrein production. The amount of sugars in the fruits has an additional impact on terrein production and could cause experimental variations between different batches of fruits. Therefore, to quantify the terrein amount on fruits of different Aspergillus terreus strains a relative production rate (in %) can be calculated by simultaneous cultivation of a well-characterised producer such as strain SBUG844 (Zaehle et al., 2014).
Recipes
- Aspergillus minimal medium plates
For 1 L:
D-Glucose10 g
20x salt stock solution (see Recipe 2)50 ml
1,000x Hutner´s trace elements (see Recipe 3)1 ml
Water900 ml
Adjust to pH 6.5 with 10 M NaOH
Agar20 g
Watermake up to 1,000 ml
Autoclave 20 min at 121 °C - 20x salt stock solution
For 1 L:
NaNO3 120 g
KCl10.4 g
MgSO4·7H2O 10.4 g
KH2PO4 30.4 g - 1,000x Hutner´s trace elements
For 100 ml:
ZnSO4·7H2O2.2 g
H3BO3 1.1 g
MnCl2·4H2O0.5 g
FeSO4·7H2O0.5 g
CoCl2·6H2O0.16 g
CuSO4·5H2O0.16 g
(NH4)6Mo7O24·4H2O0.11 g
Na2EDTA5.0 g
Heat to boiling, cool to 60 °C, add KOH adjusting pH to 6.5-6.8. The colour of the solution should turn to deep purple after storing in the dark for several days. - HPLC solvent A
HPLC pure water + 0.1% formic acid
Add 1 ml formic acid (98% purity) to 999 ml of water and filter - HPLC solvent B
Methanol (HPLC grade)
Acknowledgments
This protocol gives a detailed description of methods published previously in Gressler et al. (2015) and Zaehle et al. (2014). This work was financially supported by the German Science Foundation (DFG grant BR 2216/4-1) and internal funding from the Hans Knöll Institute, Jena (Germany).
References
- Gressler, M., Meyer, F., Heine, D., Hortschansky, P., Hertweck, C. and Brock, M. (2015). Phytotoxin production in Aspergillus terreus is regulated by independent environmental signals. Elife 4.
- Zaehle, C., Gressler, M., Shelest, E., Geib, E., Hertweck, C. and Brock, M. (2014). Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity.ChemBiol 21(6): 719-731.
Article Information
Copyright
Gressler and Brock. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gressler, M. and Brock, M. (2016). Aspergillus terreus Infection of Fruits and Terrein Quantification by HPLC Analysis. Bio-protocol 6(12): e1845. DOI: 10.21769/BioProtoc.1845.
- Gressler, M., Meyer, F., Heine, D., Hortschansky, P., Hertweck, C. and Brock, M. (2015). Phytotoxin production in Aspergillus terreus is regulated by independent environmental signals. Elife 4.07861.
Category
Microbiology > Microbe-host interactions > Fungus
Microbiology > Microbial biochemistry > Other compound
Plant Science > Plant immunity > Host-microbe interactions
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









