- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In utero Electroporation of Mouse Cerebellar Purkinje Cells
Published: Vol 6, Iss 11, Jun 5, 2016 DOI: 10.21769/BioProtoc.1835 Views: 16310
Reviewed by: Oneil G. BhalalaEmmanuelle BerretXuecai Ge

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Organotypic Explants of the Embryonic Rodent Hippocampus: An Accessible System for Transgenesis
Archana Iyer [...] Shubha Tole
Mar 20, 2018 7626 Views

Explant Culture of the Embryonic Mouse Spinal Cord and Gene Transfer by ex vivo Electroporation
Mariko Kinoshita-Kawada [...] Jane Y. Wu
Sep 20, 2019 6970 Views

Electroporation of Small Interfering RNAs into Tibialis Anterior Muscles of Mice
Anna Stephan [...] Fabio Demontis
Jun 5, 2022 3810 Views
Abstract
In utero electroporation (IUE) of mouse cerebellar Purkinje cells allows high expression levels of transgenes without toxicity (Nishiyama et al., 2012). This technique is suitable for co-transfection of multiple plasmid genes. Therefore, it is useful to express various sets of genes such as drug-inducible Cre/loxP constructs and CRISPR/Cas9 genome editing constructs (Takeo et al., 2015). Murine Purkinje cells arise from subventricular zone of fourth ventricle at embryonic day (E) 10-12. IUE at E11.5 into fourth ventricle results the most efficient transfection into Purkinje cells.
Keywords: Purkinje cellMaterials and Reagents
- Glass capillary [(Sutter Instrument Company, catalog number: BF100-50-10 ) for microinjector or (World Precision Instruments, catalog number: 1B150F-3 ) for mouth pipette]
- Sterile gauze (KAWAMOTO CORPORATION, catalog number: 7164 )
- Surgical tape (3M, catalog number: 1527SP-0 )
- Surgical scalpel blade (Swann Morton, model: No.11 )
- Suture needle (Alfresa pharma, catalog number: HT1605 NA75-KF2 )
- E11.5 (or E10~12) pregnant mouse
- QIAFilter plasmid maxi kit (QIAGEN, catalog number: 12263 )
- Fast green (Sigma-Aldrich, catalog number: F7258 )
- Sodium pentobarbital (Somnopentyl) (KYORITSU SEIYAKU CORPORATION, catalog number: 4992945014418 )
- Ethanol
- Ritodrine hydrochloride (Sigma-Aldrich, catalog number: R0758 )
- (Optional) EEG conductive paste (NIHON KOHDEN CORPORATION, catalog number: Z181JE )
- HEPES (Sigma-Aldrich, catalog number: H4034 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333 )
- Disodium hydrogenphosphate 12-water (Na2HPO4・12H2O) (Wako Pure Chemical Industries, catalog number: 196-02835 )
- Potassium dihydrogen phosphate (KH2PO4) (Wako Pure Chemical Industries, catalog number: 169-04245 )
- HEPES-buffered saline (HBS) (see Recipes)
- Phosphate-buffered saline (PBS) (see Recipes)
Equipment
- Micropipette puller (Sutter Instrument Company, model: P-87 )
- Microscope with 40x objective (Nikon Instruments Inc., model: Eclipse E100 )
- Scissors, fine forceps and ring forceps
- Square wave electroporator (Nepa Gene Co., model: CUY21SC )
- Tweezers-type electrodes (Nepa Gene Co., model: CUY650P3 )
- Microinjector (Eppendorf AG, model: 5242 ) or mouth pipette
- LED light source with flexible light guide (Kenko Tokina, model: KTX-20L )
- Reflex 7 mm wound clip applier (Cellpoint Scientific Inc., catalog number: 204-1000 )
Procedure
- DNA preparation
- Purify plasmids using the QIAGEN plasmid maxi kit. Dissolve plasmids into 1x HBS to make plasmid stock solutions. 4 mg/ml or higher concentration of plasmid stock is recommended. Plasmid stock solutions can be stored at 4 °C for several months, though fresh plasmids bring good results.
- Dilute plasmids into 30~60 μl 1x HBS at a final concentration of 2-5 mg/ml. Add 1/100 volume of 1% fast green solution.
- Purify plasmids using the QIAGEN plasmid maxi kit. Dissolve plasmids into 1x HBS to make plasmid stock solutions. 4 mg/ml or higher concentration of plasmid stock is recommended. Plasmid stock solutions can be stored at 4 °C for several months, though fresh plasmids bring good results.
- Preparation of glass micropipettes for DNA injection
- Pull glass capillaries with the micropipette puller.
- Cut the tip of a pulled pipette at 30-45 μm in diameter (Figure 1B) using a surgical scalpel blade. The cut edge must be sharpened, not a clean cut shape (Figure 1A-B). Check the cut edge under microscope. Make 3~5 glass micropipettes.
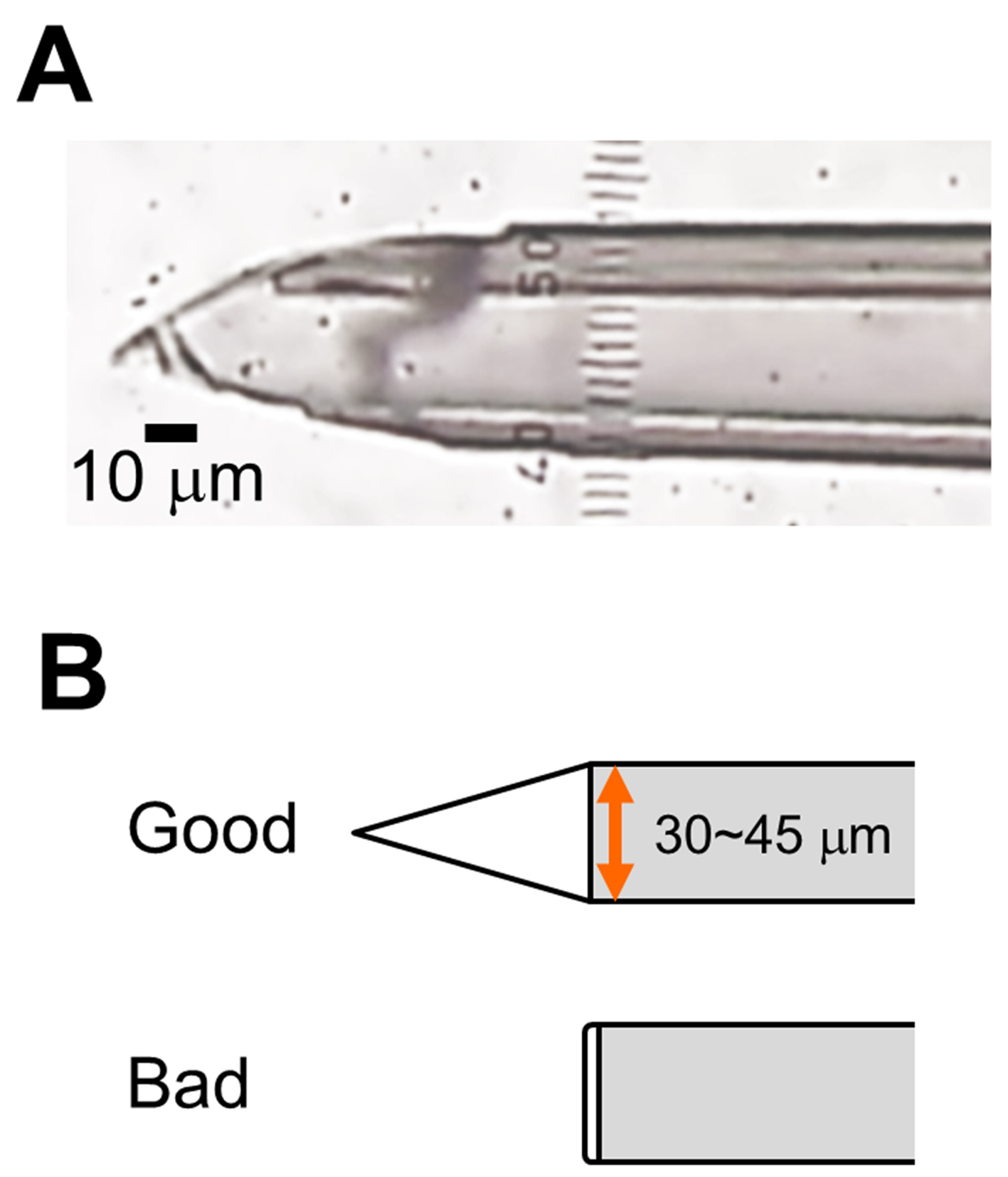
Figure 1. Glass micropipette with sharp cut edge. A. Representative image of the tip of glass micropipette (Scale bar, 10 μm). B. Schematic of the cut edge.
- Pull glass capillaries with the micropipette puller.
- Electroporation
- Anesthetize the pregnant mouse via an intraperitoneal injection of sodium pentobarbital (50-60 μg/g body weight). All procedures related to animal care and treatment were performed in accordance with the guidelines set by the Animal Resource Committee of Keio University.
- Fill the plasmid solution into glass micropipettes.
- Place the anesthetized mouse with the abdomen upside and fix the tail and limbs gently on the operation board with a surgical tape. Wipe the skin of the abdomen with 70% ethanol.
- Incise with scissors the midline of the abdomen skin, and then the abdomen wall.
- Place sterile gauze around the incision. Wet the gauze with sterile warm PBS. Apply 100-200 μl ritodrine hydrochloride solution (0.1 mg/ml) into intraperitoneal space to relax uterine muscle.
- Pinch the abdomen wall with a fine forceps at one side of the incision, insert a ring forceps into abdomen and carefully pull out one of the two uterine horns. Drop sterile warm PBS throughout the exposed uterine horn. Keep the uterus wet with PBS during surgery.
- Inject DNA solution into the fourth ventricle using the mouth pipette or microinjector (Video 1). To visualize the fourth ventricle, illuminate an embryo with the LED light from the side or underneath. Hold the embryo between the light and your fingers. Gently push the embryo so that the fourth ventricle is fixed in superficial portion of the uterus (Figure 2A-B). Insert the glass micropipette into the fourth ventricle through the uterus wall, and inject DNA by air pressure until the rostral part of fourth ventricle is filled with DNA (Figure 2C). For an embryo, take 5 seconds or longer to inject DNA solution.

Figure 2. Schematics of DNA injection into the fourth ventricle and electroporation. A. Schematic of embryo held through uterus; B. Light illumination visualize the forth ventricle; C. Schematic of fourth ventricle filled with injected DNA solution (blue color); D. Schematic of electroporation. - Wet the uterus and electrodes with warm PBS. Attach the plus end of electrodes onto the rostral edge of 4th ventricle (Video 2). Minus end is located on the back or the neck. If it is difficult to contact electrodes tightly on the uterus, put a small amount of EEG paste on the electrodes to fill the gaps between electrodes and uterus. Apply electrical pulses (33 V, with a duration of 30 ms, at intervals of 970 ms per pulse, 5 cycles). 45~50 mA current is delivered (Figure 2D). For an embryo at E10-12, apply two to four sets of electrical pulses. Too much electrical pulses might be harmful.
- Move on to the next embryo and repeat steps C7-8. When finished with one of the two uterine horns, gently put them back into abdomen using your fingers.
- Pull out the other uterine horn, just like step C7. If the uterine wall is not relaxed, drop 50 μl ritodrine hydrochloride (0.1 mg/ml) directly on the exposed uterine horn. Do steps C7-9. It is important to finish steps 6-10 within 40 min. Prolonged surgery can lead to death of the embryos or the pregnant mouse.
- After electroporated uterus are returned into abdomen, suture the wound of abdomen wall.
- Close the skin with wound clips.
- Recover the mouse under warm (~37 °C) condition. Return the mouse to the home cage.
- Electroporated mice can be analyzed at embryonic or postnatal ages (Figure 3). In the cerebellar vermis which was electroporated at E11.5, 95% of the transfected cells are Purkinje cell.
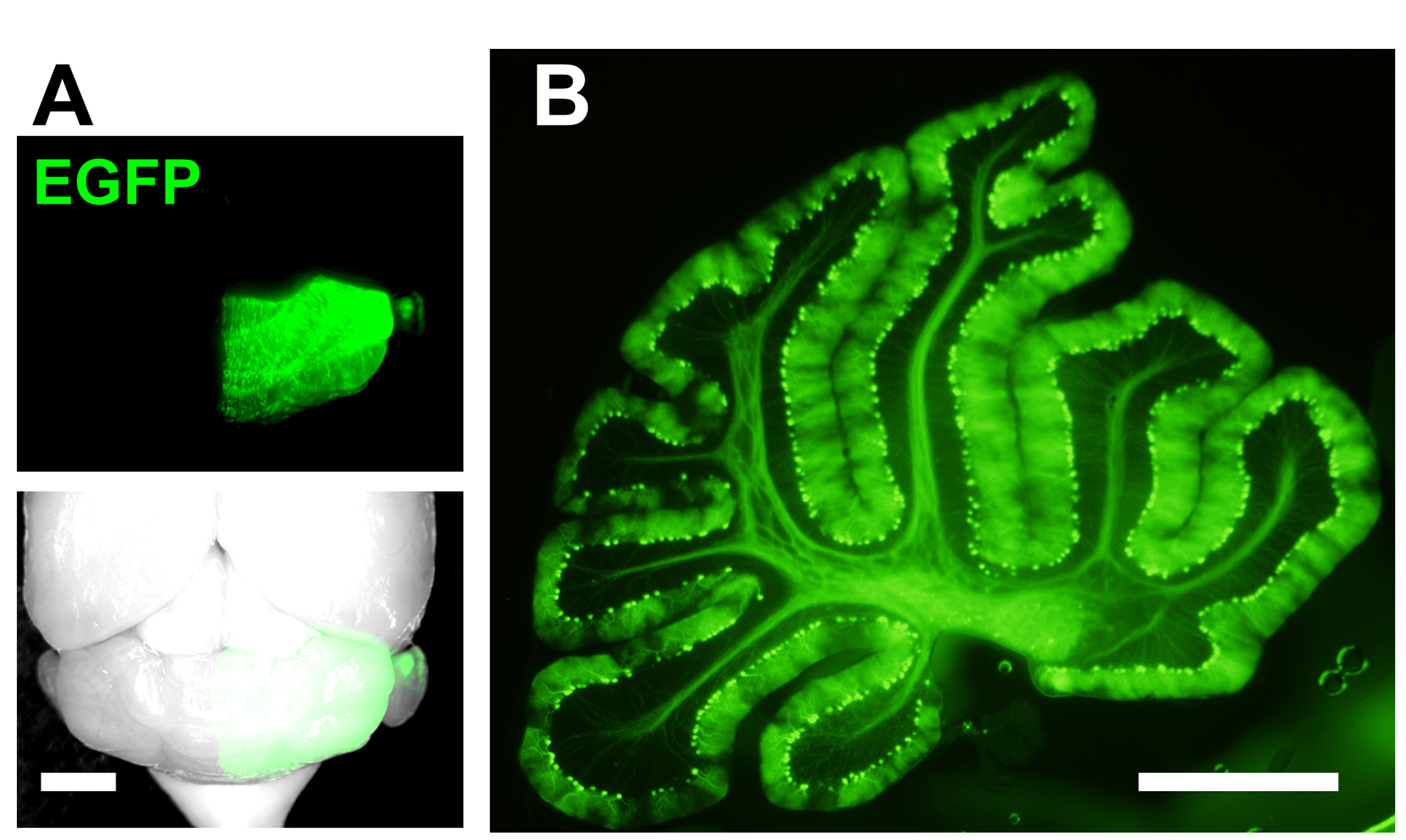
Figure 3. Representative result of IUE. E11.5 mouse was electroporated with EGFP-encoding plasmids and analyzed at P33. A. Mosaic labeling of Purkinje cells by EGFP is observed in right half of the cerebellum (Scale bar, 2 mm). In this example, the plus end of the electrodes was placed on the right side of the subventricular zone during electroporation; B. Sagittal slice of the electroporated cerebellum shown in A (Scale bar, 1 mm).
- Anesthetize the pregnant mouse via an intraperitoneal injection of sodium pentobarbital (50-60 μg/g body weight). All procedures related to animal care and treatment were performed in accordance with the guidelines set by the Animal Resource Committee of Keio University.
Representative data
Recipes
- HEPES-buffered saline (HBS)
21 M HEPES
137 mM NaCl
5 mM KCl
0.7 mM Na2HPO4 - Phosphate-buffered saline (PBS) [Sterile and pre-warmed (37 °C)]
137 mM NaCl
2.7 mM KCl
8.1 mM Na2HPO4
1.47 mM KH2PO4
Acknowledgments
This protocol was adapted from Nishiyama et al. (2012). This work was supported by Japan Society for the Promotion of Science.
References
- Nishiyama, J., Hayashi, Y., Nomura, T., Miura, E., Kakegawa, W. and Yuzaki, M. (2012). Selective and regulated gene expression in murine Purkinje cells by in utero electroporation. Eur J Neurosci 36(7): 2867-2876.
- Takeo, Y. H., Kakegawa, W., Miura, E. and Yuzaki, M. (2015). RORalpha regulates multiple aspects of dendrite development in cerebellar purkinje cells in vivo. J Neurosci 35(36): 12518-12534.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Takeo, Y. H. (2016). In utero Electroporation of Mouse Cerebellar Purkinje Cells. Bio-protocol 6(11): e1835. DOI: 10.21769/BioProtoc.1835.
- Takeo, Y. H., Kakegawa, W., Miura, E. and Yuzaki, M. (2015). RORalpha regulates multiple aspects of dendrite development in cerebellar purkinje cells in vivo. J Neurosci 35(36): 12518-12534.
Category
Neuroscience > Development > Electroporation
Cell Biology > Tissue analysis > Electroporation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link