- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Micro Neutralization (MN) Assay of Influenza Viruses with Monoclonal Antibodies
(*contributed equally to this work) Published: Vol 6, Iss 11, Jun 5, 2016 DOI: 10.21769/BioProtoc.1829 Views: 16187
Reviewed by: Ivan ZanoniMigla MiskinyteAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Nab Escaping AAV Mutants Isolated from Mouse Muscles
Zheng Chai [...] Chengwen Li
May 5, 2018 7593 Views

A Refined Protocol for Identifying Citrulline-specific Monoclonal Antibodies from Single Human B Cells from Rheumatoid Arthritis Patient Material
Khaled Amara [...] Caroline Grönwall
Sep 5, 2019 6995 Views

Multiplication and Growth Inhibition Activity Assays for the Zoonotic Malaria Parasite, Plasmodium knowlesi
Franziska Mohring [...] Robert W. Moon
Sep 5, 2020 5896 Views
Abstract
The human monoclonal antibodies generated from single human B cells were tested to characterize their ability to neutralize virus infectivity. The microneutralization assay is a highly sensitive and specific assay for detecting virus-specific neutralizing antibodies to influenza viruses. This protocol is to measure the ability of human monoclonal antibody to neutralize influenza virus by microneutralization assay.
Materials and Reagents
- Materials
- 96 well microtiter plates (Corning, catalog number: 3595 )
- Tips for multichannel pipette (Gilson, model: D10 , D200 and D1000 )
- Centrifuge tubes (SARSTEDT AG & Co, catalog number: 72.690 )
- 96 well microtiter plates (Corning, catalog number: 3595 )
- Reagents
- Viral antigen
Note: Viruses were amplified in embryonated eggs or Madin–Darby canine kidney (MDCK) cells. Refer to Manual for the laboratory diagnosis and virological surveillance of influenza, WHO.- H1N1
A/Ohio/83
A/Solomon Islands/2006
A/Ohio/07/2009
A/Texas/05/2009-RG15
A/Texas/18/2009-RG18
A/California/04/2009 - H2N2
A/Ann Arbor/6/60 ca - H5N1
A/Vietnam/1203/04 (VNH5N1-PR8/CDC-RG)
A/Anhui/01/2005(H5N1)-PR-IBCDC-RG6 - H9N2
A/ck/HK/G9/97(H9N2)/PR8-IBCDC-2
A/Green-winged teal/209/TX/2009 - H3N2
A/Hong Kong/68
A/Philippines/2/1982
A/Beijing/353/89-X109-H3N2 PR8 reassortant
A/Beijing/32/92-R-H3N2 PR8 reassortant
A/Johannesburg/33/94 R-H3N2 PR8 reassortant
A/Nanchang/933/95
A/Sydney/5/97
A/Panama/2007/99
A/Wyoming/3/03.rg
A/Brisbane/10/07 - H7N2
A/turkey/Virginia/2002(H7N2)/ PR8-IBCDC-5 - H7N9
A/Anhui/1/2013
A/Shanghai/2/2013
- H1N1
- Cells
Madin-Darby canine kidney (MDCK) cells (ATCC, catalog number: CCL-34 ) - Neutralization antibody
Human monoclonal antibody, CT149 (Celltrion INC., South Korea) from convalescent patients infected with A(H1N1)pdm09 - Media
- DMEM (Invitrogen, catalog number: 11965-092 )
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 11965-092”. - Fetal bovine serum (FBS) (VWR International, Hyclone™, catalog number: SH30070.03 )
- Penicillin-streptomycin (Invitrogen, catalog number: 15140-122 )
Note: Currently, it is (Thermo Fisher Scientific, Gibco™, catalog number: 15140-122)”. - L-glutamine, 200 mM solution (Invitrogen, catalog number: 25030-081 )
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 25030-081”. - Bovine serum albumin (BSA) (fraction V, protease free) (Roche Diagnostics, catalog number: 0 3117332001 )
- HEPES, 1 M Buffer Solution (Invitrogen, catalog number: 15630-080 )
Note: Currently, it is (Thermo Fisher Scientific, Gibco™, catalog number: 15630-080)”.
- DMEM (Invitrogen, catalog number: 11965-092 )
- Other reagents
a.Antibody for ELISA- Primary antibodies [Anti-nucleoprotein (NP) antibodies], Mouse Anti-Influenza A antibody (Merck Millipore Corporation, catalog number: MAB8257 ) and Anti-Influenza A antibody (Merck Millipore Corporation, catalog number: MAB8258 )
- Secondary antibody, goat anti-mouse IgG conjugated to horseradish peroxidase (HRP) (Kirkegaard & Perry Laboratories, Inc., catalog number: 074-1802 )
c.Tween-20 (Merck Millipore Corporation, catalog number: 8.17072 )
d.Antibody diluent (Teknova, catalog number: D5120 )
e.Acetone (Sigma-Aldrich, catalog number: 270725 )
f.3, 3’, 5, 5’-Tetramethylbenzidine (TMB) (Sigma-Aldrich, catalog number: T0440 )
g.Stop solution (Merck Millipore Corporation, catalog number: 109072 )
Note: It is named “Sulfuric acid” on Merck Millipore Corporation website. - Primary antibodies [Anti-nucleoprotein (NP) antibodies], Mouse Anti-Influenza A antibody (Merck Millipore Corporation, catalog number: MAB8257 ) and Anti-Influenza A antibody (Merck Millipore Corporation, catalog number: MAB8258 )
- Wash buffer (see Recipes)
- MDCK medium (see Recipes)
- Virus diluent media (see Recipes)
- Viral antigen
Equipment
- Haemacytometer (Hausser Scientific, catalog number: 1492 )
- Multichannel pipette (Gilson, model: PIPETMAN Neo® Multichannel )
- Incubator, 37 °C, 5% CO2 (Panasonic Corporation, Sanyo Electronics company, model: MCO-170AIC-PE )
- SpectraMax M5 multi-detection microplate reader system (Molecular Devices, model: M5 )
Procedure
- Prepare initial monoclonal antibody dilutions using virus diluent.
- Add 50 μl of virus diluent to all wells in column 1 to 10.
- Add 50 μl of the monoclonal antibody (1 mg/ml stock solution) to the first well and make 2-fold serial dilutions by transferring 50 μl using a multi-channel pipette from the first well to each successive well from 1:1 to 1:128. Refer to Figure 1.
- Discard 50 μl after the last dilution.
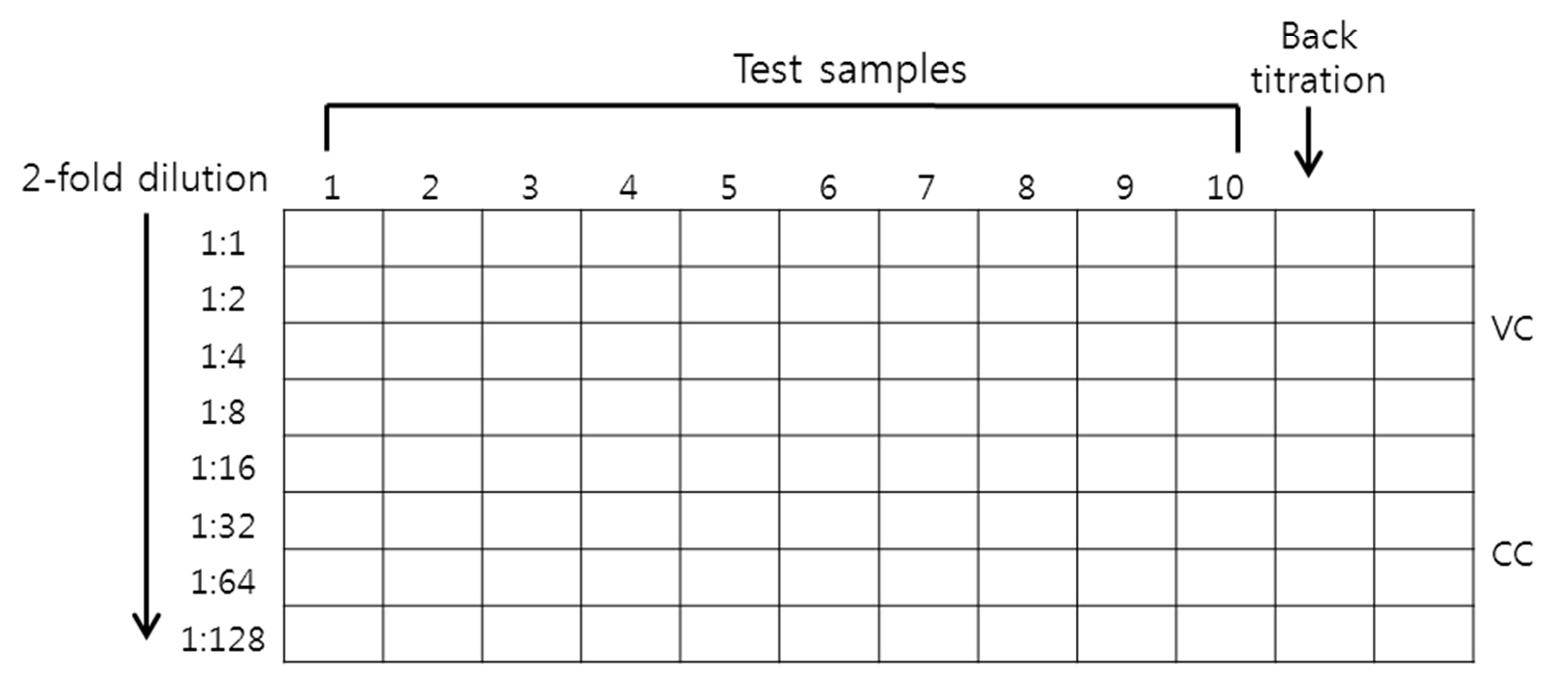
Figure 1. Serial dilution of test sample for MN assay. Add 50 μl of virus diluent to all wells in column 1 to 10 and add 50 μl of the sample to the first well and make 2-fold serial dilutions by transferring 50 μl using a multi-channel pipette. Dilution factor of sample is 1 to 128. - Cover and hold in 37 °C, 5% CO2 incubator while the diluted virus is being prepared. It is critical that the proper pH is maintained so that there will be no deleterious pH effects on the virus when it is added.
- Dilute the virus in virus diluent to the correct working dilution as 100 TCID50 /50 μl (TCID50: 50% Tissue culture infective dose, 100 TCID50: 100x TCID50).
- Add 50 μl of diluted virus to wells containing antibodies and the virus control (VC) wells (A12, B12, C12 and D12) and do not add virus to the cell control (CC) wells (E12, F12, G12 and H12) and do not add virus to the column of wells reserved for the virus back titration.
- Figure 2 showed detailed diagram for serial dilution of test antibody and back titration.
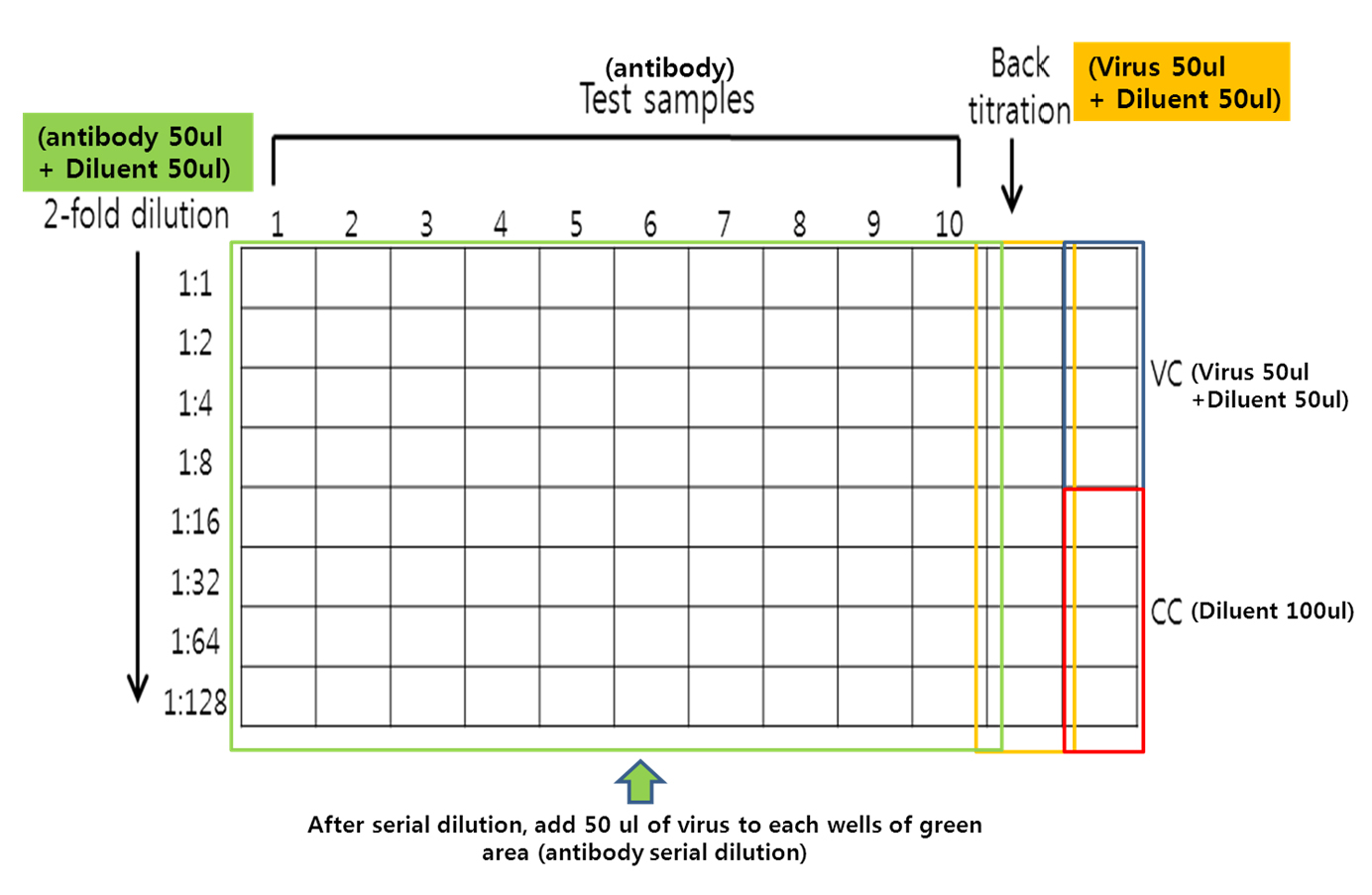
Figure 2. Serial dilution of antibody and back titration. Serial dilution of test sample (green box) and virus back titration (yellow box) is 2-fold dilution by 50 μl + 50 μl. Virus control (blue box) is also 50 μl + 50 μl, but cell control (red box) is only 100 μl of diluent. - Tap the plate gently to mix.
- Add 100 μl of virus diluent to the CC wells.
- Add 50 μl of virus diluent to the VC wells.
- In each assay, include a back titration of the working dilution of virus because neutralizing antibody titer is changed sensitively depend on virus titer.
- Add 50 μl of virus diluent to all wells in column 11.
- Add 50 μl of the working dilution of virus to the first well and make 2-fold serial dilutions by transferring 50 μl using a multi-channel pipette from the first well to each successive well through well 12.
- Discard 50 μl from well 12.
- Add additional 50 μl of virus diluent to all wells for total volume to 100 μl.
- Cover and incubate at 37 °C, 5% CO2 for 1 h.
- After 1 h, add 100 μl of diluted MDCK cells (which should be 70-95% monolayer and low passage <30) to each well of microtiter plates. Each well contains 1.5 x 104 cells.
- Incubate at 37 °C, 5% CO2 for 18-20 h.
- After incubation, remove the medium from microtiter plates and wash the plates with 200 μl of PBS and then remove PBS (overturn the plate by hand to spill the PBS and then swap remaining liquid using paper towel).
- Add 100 μl of 80% cold acetone to each wells and incubate at room temperature (RT) for 10-12 min for fixation before ELISA.
- Remove the acetone (overturn the plate by hand to spill the acetone and then swap remaining liquid using paper towel), let the plates air-dry for 10 min or until dry.
- Dilute the primary antibody in antibody diluent to an optimum working dilution.
- Wash the plate 3x with 300 μl of the wash buffer and add 100 μl of diluted primary antibody (1:1,000).
- Cover and incubate for 1 h at RT.
- Dilute the secondary antibody in antibody diluent to an optimum working dilution
- Wash the plate 3x with 300 μl of the wash buffer and add 100 μl of diluted secondary antibody (1:2,000).
- Cover and incubate for 1 h at RT.
- Wash the plate 5x with 300 μl of the wash buffer and add 100 μl of freshly prepared TMB substrate.
- Incubate at RT until the color change in the VC wells is intense and the corresponding color change in the CC wells in minimal.
- Add 100 μl of stop solution.
- Read the absorbance (O.D.) of wells at 490 nm using microtiter plate spectrophotometer.
- The O.D. in the VC wells should be at least 0.8 and is typically in the range 1.0-1.5 though higher is acceptable.
- The O.D. in the CC wells must ≤ 0.2.
- The data are analyzed as follows.
- Evaluated to determine if the working dilution of virus used in the assay contained the desired amount of virus. The cut-off value for the virus back titration is the mean of the VC median and the CC median. This is the same cut-off value that is used to calculate neutralizing antibody titers. The dilution of the first well below the cut-off value is the back titration titer. The dilutions in the back titration wells are beginning at well A: 1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128. In general, back titration titers of 16, 32, and 64 are acceptable.
Example of back titration
Median O.D. of CC = 0.2
Median O.D. of VC= 1.0
Mean of the VC median and the CC median = (0.2+1.0)/2= 0.6 = cut-off value
O.D. of back titration wells (Table 1)
Table 1. Example of O.D. of back titration wellsDilution 1:1 1:2 1:4 1:8 1:16 1:32 1:64 1:128 O.D. 2 1.5 1 0.8 0.5 0.4 0.3 0.2
1:16 (O.D.: 0.5) is the first well below the cut-off value (O.D. 0.6).
Back titration titer is 1:16. - Neutralizing antibody titers are determined by calculating the cut-off value to determine a 50% neutralizing antibody titer for each plate based on the equation: (median O.D. of VC + median O.D. of CC)/2 = X, where X = the 50% cut-off value. All values below or equal to X are positive for neutralization. Read each column which contained diluted antibody from the bottom, beginning at well H. Note the first well with an OD of less than the 50% cut-off. The reciprocal antibody dilution corresponding to that well is the 50% neutralization antibody titer for that antibody sample.
Example of neutralizing antibody titer
Median O.D. of CC = 0.2
Median O.D. of VC = 1.0
Mean of the VC median and the CC median = (0.2+1.0)/2 = 0.6 = cut-off value
O.D. of back titration wells (Table 2)
Table 2. Example of O.D. of neutralizing antibody wellsDilution 1:1 1:2 1:4 1:8 1:16 1:32 1:64 1:128 O.D. 0.1 0.2 0.3 0.4 0.5 0.8 1.5 2.0
1:4 (O.D.: 0.5) is the first well below the 50% cut-off value (O.D. 0.6).
Neutralizing antibody titer is 1:16.
- Evaluated to determine if the working dilution of virus used in the assay contained the desired amount of virus. The cut-off value for the virus back titration is the mean of the VC median and the CC median. This is the same cut-off value that is used to calculate neutralizing antibody titers. The dilution of the first well below the cut-off value is the back titration titer. The dilutions in the back titration wells are beginning at well A: 1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128. In general, back titration titers of 16, 32, and 64 are acceptable.
Recipes
- Wash buffer
Phosphate buffered saline (PBS) with 0.3% Tween 20 - MDCK medium
DMEM supplemented with
10% fetal bovine serum (FBS)
100 U/ml penicillin
100 mg/ml streptomycin
2 mM L-glutamine
Sterilize by filtration - Virus diluent media
DMEM, supplemented with 1% bovine serum albumin (BSA) (fraction V, protease free) (prepared as a 10% w/v solution in dH2O, filter sterilized, and stored at 4-8 °C)
100 U/ml penicillin
100 mg/ml streptomycin
20 mM HEPES
Prepare fresh for each assay
Sterilized by filtration
Acknowledgments
We thank Dr. Jun Myung Kim and Dr. Sang Hoon Han for providing blood samples from Severance Hospital, and Dr. Yasuo Watanabe of SC World, Inc. for assistance in ISAAC assays for antibody screening. This study was supported by a grant of the Korea Healthcare technology R&D Project‚ Ministry of Health&Welfare‚ Republic of Korea. (Grant No. A103001), the National Natural Science Foundation of China (NSFC, Grant No. 81401671), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB08020100), the China National Grand S&T Special Project (Grant No. 2015ZX09304005) and G. F. G. is a leading principal investigator of the NSFC Innovative Research Group (Grant No. 81321063). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
References
- Klimov, A., Balish, A., Veguilla, V., Sun, H., Schiffer, J., Lu, X., Katz, J. M. and Hancock, K. (2012). Influenza virus titration, antigenic characterization, and serological methods for antibody detection. Methods Mol Biol 865: 25-51.
- Manual for the laboratory diagnosis and virological surveillance of influenza. WHO global influenza surveillance network.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wu, Y., Cho, M., Shore, D., Song, M., Choi, J., Jiang, T., Deng, Y., Bourgeois, M., Almli, L., Yang, H., Chen, L., Shi, Y., Qi, J., Li, A., Yi, K. S., Chang, M., Bae, J. S., Lee, H., Shin, J., Stevens, J., Hong, S., Qin, C., Gao, G. F., Chang, S. J. and Donis, R. O. (2016). Micro Neutralization (MN) Assay of Influenza Viruses with Monoclonal Antibodies. Bio-protocol 6(11): e1829. DOI: 10.21769/BioProtoc.1829.
Category
Immunology > Antibody analysis > Antibody function
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









