- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolating Taste Buds and Taste Cells from Vallate Papillae of C57BL/6J Mice for Detecting Transmitter Secretion
Published: Vol 6, Iss 11, Jun 5, 2016 DOI: 10.21769/BioProtoc.1824 Views: 9303
Reviewed by: Soyun KimRenate WeizbauerGeoff Lau

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Mouse is a well-accepted model for studying taste bud function. Mice readily detect and respond to taste substances that humans consider to have sweet, bitter, salty, sour and umami taste qualities. A great deal of recent research on taste receptors is based on this species. Live mice are needed for these experiments because no alternative in vitro model incorporates all elements of taste transduction and peripheral signaling. The C57BL/6J strain was selected because these mice respond robustly to many taste stimuli and because of variety of transgenic animals, such as PLCβ2-GFP and GAD67-GFP, were derived from that strain. Prior analyses on behavior, nerve responses, cellular electrophysiology and molecular biology, all conducted on C57BL/6J mice will form a solid foundation for the proposed studies (Finger et al., 2005; Huang and Wu, 2015; Huang et al., 2007). Thus, freshly euthanized animals must be used as a source of taste buds from which we will isolate taste buds and taste cells.
Keywords: Taste budMaterials and Reagents
- Fire-polished borosilicate glass micropipette (World Precision Instrument) with suction apparatus (Figure 1D)
- Polyethylene tubing (BD, Intramedic™, model: PE#205 )
- 1 ml syringe (BD)
Caution: Pipette tip must be large enough to allow taste buds to easily pass through the opening (60~80 µm and 20~30 µm for taste buds and taste cells collection, respectively). However, a wide opening will result in a large volume of solution being drawn into the suction pipette.
- Polyethylene tubing (BD, Intramedic™, model: PE#205 )
- Sylgard dish with dissecting pins (Figure 2A)
- Eppendorf centrifuge tubes (1.5 ml)
- 35 mm culture dishes (Corning)
- Plastic two-way valve of syringes (Figure 1D)
- Cell-TAK cell and tissue adhesive (Corning) coated coverslip(s)
Caution: Apply a tiny drop of Cell-TAK onto the center of 12-mm coverslips. Wait until the droplet is completely dry. Use ethanol (75%) followed by nanopure UV water to rinse and clean coverslips. - C57BL/6J strain mice (the Jackson laboratory) (Figure 1A)
- 100% CO2
- Nanopure UV water (Thermo Fisher Scientific)
- Collagenase A (Roche Diagnostics, catalog number: 10103578001 )
- Dispase II (Roche Diagnostics, catalog number: 04942078001 )
- Elastase (Worthington Biochemical Corporation, catalog number: 2292 )
- Trypsin inhibitor (Roche Diagnostics, catalog number: 10109886001 )
- Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C5670 )
- HEPES (Sigma-Aldrich, catalog number: H4034 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P5405 )
- Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M4880 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S6297 )
- Sodium pyruvate (Sigma-Aldrich, catalog number: P5280 )
- D-(+)-Glucose (Sigma-Aldrich, catalog number: G7021 )
- BAPTA (EMD Millipore Corporation, Calbiochem, catalog number: 196418 )
- Ethylene glycol-bis(2-aminoethylether)-N, N, N’, N’-tetraacetic acid (EGTA) (Sigma-Aldrich, catalog number: E0396 )
- Enzyme mixture (see Recipes)
- Tyrode’s buffer (see Recipes)
- Ca2+/Mg2+-free Tyrode’s buffer (see Recipes)
Equipment
- Olympus IX73 inverted fluorescence microscope (Olympus Imaging America Inc., Olympus Optical)
- Stereo dissecting microscope (ZEISS, ZEISS Optical) (Figure 1C)
- Fiber optical illuminator (Dolan-Jenner Industrial Inc.)
- Compressed CO2 gas in the cylinder (Figure 1B)
- Plastic chamber connecting with an ample length of vinyl tubing
- XSE analytical balance (Mettler-Toledo International Inc.)
- Single-sample micro osmometer (Advanced Instruments, model: 3320 )
- Thermo Scientific benchtop pH meter (Thermo Fisher Scientific, Accumet™, model: XL15 )
- Narishige microforge for fire polishing with tungsten filament, electrically heated (NARISHIGE Group, model: MF-900 )
- Flaming/Brown glass micropipette puller (Sutter Instruments, model: P-1000 )
- Recording/Perfusion chamber (Warner Instruments, model: RC-25 ) (Figure 2B)
- Magnetic platform (PH4) for RC-25 recording chamber, and for mounting onto an Olympus IX73 microscope (Figure 2B)
- Thermolyne stirrer (Thomas Scientific, model: Nuova II )
- Spectrafuge 7M microcentrifuge (ReGen Lab Equipment, model: SKU: W095074 )
- Eppendorf 5702 centrifuge (Thermo Fisher Scientific, Fisher ScientificTM, model: Eppendorf 5702 )
- VWR vortex mixer for vortexing the enzyme mix (VWR International, model: MINI 230V )
- Gilson pipettors (200 μl and 1 ml tips) (VWR International)
- Sharp tweezers
- Microsurgical scissors
Procedure
- Mice are euthanized by cervical dislocation under deep CO2 (100%) anesthesia (Figure 1A-B).
- Obtain the tongue from animals and place in Tyrode’s buffer.
- Pin tongue on a Sylgard dish so that vallate papillae are easily accessible (Figure 1C).
- Inject ~0.3 ml of enzyme mixture in the regions near the vallate papillae (on each side) subepithelially. The needle should be visibly beneath the epithelium.
Note: Delivering enzyme mixture by inserting the needle too deep will result in the digestion of the muscle tissue. If the location of needle is too shallow, the epithelium will be pierced, the enzyme mixture may leak through the holes on the lingual epithelium.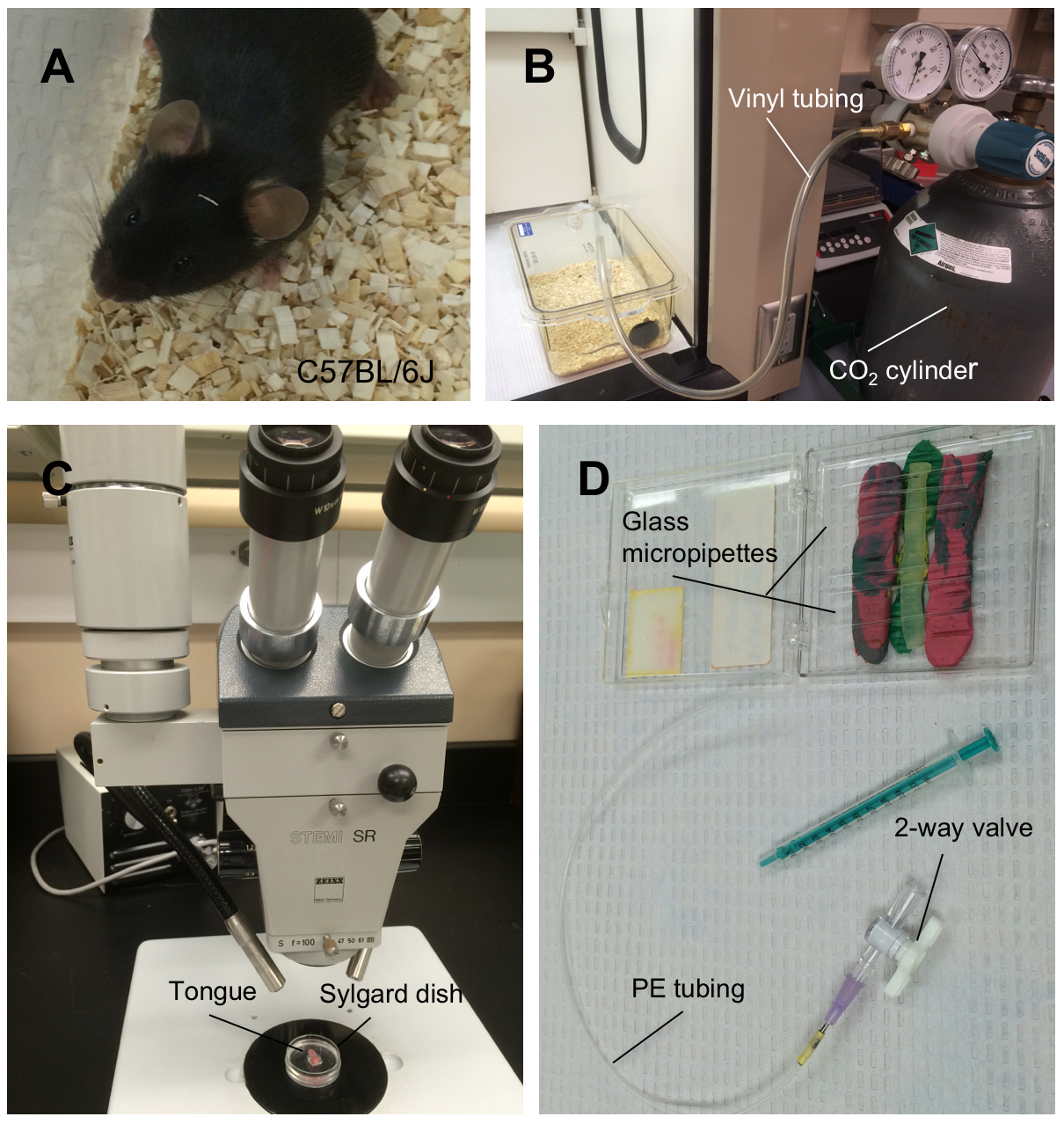
Figure 1. Euthanization of mice, and excision of tongues. The photograph shows (A) the animal, C57BL/6J mouse, (B) the apparatus for euthanizing animals, (C) the tongue pinned on a Sylgard dish under the stereo dissecting microscope, and (D) the suction apparatus for collecting taste buds. - Incubate the tongue in 5~10 ml of Tyrode’s buffer with aeration for 30 min.
- Re-pin the tongue so that vallate papillae are readily accessible (Figure 1C).
- Cut the epithelium surrounding vallate papillae with microsurgical scissors.
- Peel the epithelium away from the tongue using a sharp set of tweezers.
- Pin the pieces of epithelium, serous side up, onto a Sylgard dish. Stretch the epithelium so that each papilla is exposed and easily accessible (Figure 2A).
- Exchange the solution for Ca2+/Mg2+-free Tyrode’s buffer. Incubate the epithelium in the buffer for 20 min for collecting individual taste buds or 30 min for single taste cells.
- Replace Ca2+/Mg2+-free Tyrode’s buffer in the Sylgard dish with regular Tyrode’s buffer.
- Using a fire-polished glass micropipette (60~80 μm) (Figure 1D), sweep each papilla several times while applying suction. Periodically stop and discharge the solution onto a Cell-TAK treated coverslip.
Critical: There are, typically, sufficient number of taste buds on the vallate papillae to make 2~4 coverslips.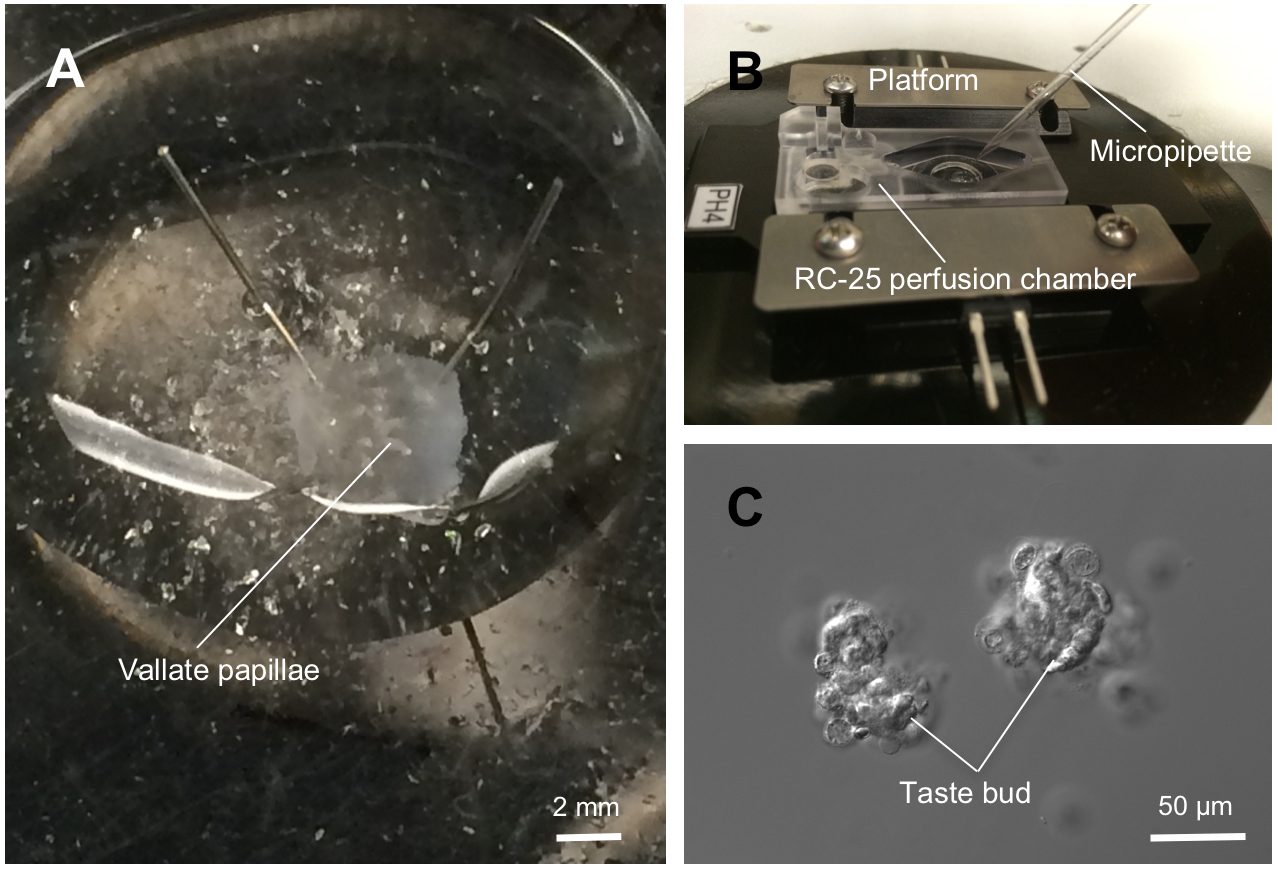
Figure 2. Suction apparatus setup to collect taste buds from mouse vallate papillae. A. The photo shows the peeled lingual epithelium, serous side up, pinned on a Sylgard dish. B. Isolation of taste buds is performed in the Sylgard dish, and then taste buds are delivered via a glass micropipette to a recording chamber assembled on the platform. C. A Nomarski optics micrographic image of isolated taste buds in a living preparation when viewed under the Olympus IX73 microscope. - For collecting single cells, gentle trituration (suck Tyrode’s containing taste buds into the glass pipette and discharge it immediately for repeating 5-6 times) is required via 20~30 μm fire-polished glass pipettes.
Note: Validating the intact membranes can be a criterion for healthy-looking taste buds/cells. Taste cells may be not healthy if the intracellular Ca2+ concentration is over 200 nM at their resting stage (Critical!). - Allow 20 min for taste buds and/or taste cells to settle, and to attach to the bottom of the chamber, which is made by glass coverslips (Figure 2B-C).
Recipes
- Enzyme mixture
Collagenase A1 mg/ml
Dispase II2.5 mg/ml
Elastase1 mg/ml
Trypsin inhibitor1 mg/ml - Tyrode’s buffer
310-320 Osm and applied at pH 7.2
CaCl2 2 mM
HEPES10 mM
KCl5 mM
MgCl2 1 mM
NaCl140 mM
NaHCO3 10 mM
Na-pyruvate10 mM
Glucose10 mM - Ca2+/Mg2+-free tyrode’s buffer (pH 7.2)
CaCl2 0 mM
HEPES10 mM
KCl5 mM
MgCl2 0 mM
NaCl140 mM
NaHCO3 10 mM
Na-pyruvate10 mM
Glucose10 mM
BAPTA2 mM
EGTA2 mM
Acknowledgments
This work was supported by SIUSOM research seed grant (AYH).
References
- Finger, T. E., Danilova, V., Barrows, J., Bartel, D. L., Vigers, A. J., Stone, L., Hellekant, G. and Kinnamon, S. C. (2005). ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310(5753): 1495-1499.
- Huang, A. Y. and Wu, S. Y. (2015). Calcitonin gene-related peptide reduces taste-evoked ATP secretion from mouse taste buds. J Neurosci 35(37): 12714-12724.
- Huang, Y. J., Maruyama, Y., Dvoryanchikov, G., Pereira, E., Chaudhari, N. and Roper, S. D. (2007). The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A 104(15): 6436-6441.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Huang, A. Y. and Wu, S. Y. (2016). Isolating Taste Buds and Taste Cells from Vallate Papillae of C57BL/6J Mice for Detecting Transmitter Secretion. Bio-protocol 6(11): e1824. DOI: 10.21769/BioProtoc.1824.
-
Huang, A. Y. and Wu, S. Y. (2015). Calcitonin gene-related peptide reduces taste-evoked ATP secretion from mouse taste buds. J Neurosci 35(37): 12714-12724.
Category
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link