- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Bioassays to Investigate the Effects of Insect Oviposition on a Plant’s Resistance to Herbivores
Published: Vol 6, Iss 11, Jun 5, 2016 DOI: 10.21769/BioProtoc.1823 Views: 13338
Reviewed by: Marisa RosaJihyun KimAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Maize Seedlings Colonization with Serendipita indica and Its Colonization Efficiency Analysis
Om Prakash Narayan [...] Atul Kumar Johri
Oct 20, 2023 2276 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views
Abstract
Plants respond to herbivory with diverse defence responses (Schoonhoven et al., 2005). Many herbivorous insects deposit their eggs on their host plants before their larvae start to feed. Thus, plants could use insect eggs as a signal to increase their resistance to herbivores. Here, we report experimental procedures to explore whether and how insect oviposition impacts on plant resistance against the feeding larvae. The described approach revealed that Nicotiana attenuata (N. attenuata) plants that were previously exposed to oviposition by lepidopteran moths respond to herbivory by generalist Spodoptera exigua (S. exigua) and specialist Manduca sexta (M. sexta) larvae with an increased induction of defence responses, which results in a decreased performance or immune state of the feeding larvae (Bandoly et al., 2015; Bandoly et al., 2016). Consequently, insect oviposition can prime feeding-induced plant defence (priming: an enhanced plant response to stress upon the experience of a prior stimulus; Hilker et al., 2015). Full-factorial experiments with standardised procedures for insect oviposition and larval herbivory allow to decipher the effect of the plant exposure to insect eggs on the larval performance, feeding damage and immune state as well as to discriminate egg-induced plant responses from egg-primed responses to larval feeding.
Materials and Reagents
- Reaction tubes, 0.2 ml (e.g., Carl Roth GmbH + Co. KG, catalog number: H560.1 )
- Reaction tubes, 1.5 ml or 2 ml (e.g., Carl Roth GmbH + Co. KG, catalog number: CK06.1 )
- Nicotiana attenuata Torr. ex. Watson (Solanaceae), 4-5 weeks old (rosette stage)
- Spodoptera exigua Hübner (Noctuidae, Lepidoptera) or Manduca sexta Linnaeus (Sphingidae, Lepidoptera)
- Liquid nitrogen
Note: All materials and equipment used is rather general lab equipment or houseware and we just referred to example products we used.
Equipment
- General equipment
- A soft paint brush [e.g., Rotmarder 122A, size 2 (Habico, catalog number: 50122A10 )]
- Featherweight forceps (e.g., Carl Roth GmbH + Co. KG, catalog number: AN00.1 )
- Rearing boxes (e.g., 14 x 21 x 5 cm), lid with gauze (nylon mesh 0.12 mm width)
- Precision balance (e.g., Sartorius AG, model: Sartorius MC210S )
- A soft paint brush [e.g., Rotmarder 122A, size 2 (Habico, catalog number: 50122A10 )]
- Oviposition on plants
- Flight cages [e.g., kweekkooi, 60 x 60 x 90 cm (Vermandel, catalog number: 80.304 ) or flexarium, 42 x 42 x 76 cm (Rolf C. Hagen Inc., Exo Terra, model: PT2552 )]
Note: Flight cages eventually with slots at the cage sides through which defined leaves can be exposed and that can be closed with small claw hair clips. - Hanging labels [e.g., Hängeetiketten, HERMA FACHSHOP, model: 6901 ]] or a thread
- Headlamp (e.g., Petzl, model: PIXA® 3 )
- Flight cages [e.g., kweekkooi, 60 x 60 x 90 cm (Vermandel, catalog number: 80.304 ) or flexarium, 42 x 42 x 76 cm (Rolf C. Hagen Inc., Exo Terra, model: PT2552 )]
- Larval performance, feeding damage and haemolymph sampling
- Vented clip cages [e.g., made of two dressing cups (e.g., Ø 7.03, 2.3 cm (KIV-KREIS, catalog number: 770405509 )) with polyurethane foam at the rims, the bottom of one cup is replaced by gauze (nylon mesh 0.12 mm width) and the cage can be closed with small claw hair clips]
- Folding magnifier [e.g., TRIPLET 20x, 21 mm (Light In The Box Ltd., catalog number: 01239576 )]
- Laboratory support stand with clamps
- Whiteboard with reference areas (e.g., a laminated sheet of white paper with black squares of 1 x 1 cm)
- Camera [e.g., Canon EOS 1200D (Canon Inc., model: Canon EOS 1200D ) with EFS 18-25 mm macro lenses (Canon Inc., model: Canon EF-S 18-55mm f/3.5-5.6 IS II)]
- Vented clip cages [e.g., made of two dressing cups (e.g., Ø 7.03, 2.3 cm (KIV-KREIS, catalog number: 770405509 )) with polyurethane foam at the rims, the bottom of one cup is replaced by gauze (nylon mesh 0.12 mm width) and the cage can be closed with small claw hair clips]
- Standardised damage and harvest of leaf tissue for analyses of plant defence traits
- Vacuum pump (e.g., Vacuubrand, model: PC 510 )
- Teflon tube [e.g., outer Ø 1.6 mm (VWR International, catalog number: BOHLS1810-01 )]
- Glass vial with rubber septum cap [e.g., 4 ml HPLC vial (Techlab, catalog number: FLK 101.243 and FLK 101.972 )]
- Tracing/Pattern wheel (e.g., Nähmit, catalog number: 070210 )
- 10 µl pipette
- Scalpel (e.g., Carl Roth GmbH + Co. KG, catalog number: X004.1 )
- Dissecting forceps (e.g., Carl Roth GmbH + Co. KG, catalog number: 2690.1 )
Procedure
- Oviposition on plants
- Sort S. exigua or M. sexta pupae for their sex. Figure 1 depicts the discrimination characteristics between male and female lepidopteran pupae.
- Let the pupae eclose in the flight cages used for oviposition. In case of S. exigua place 15 female and 15 male pupae in a flight cage. In case of M. sexta place 8 female and 8 male pupae in a flight cage. Inspect the cages twice per day (in the morning and in the evening) for eclosed moths. To ensure that the moths had time to mate and have started to lay eggs use the cages 2 d after eclosion for oviposition in case of S. exigua and 3 days after eclosion in case of M. sexta.
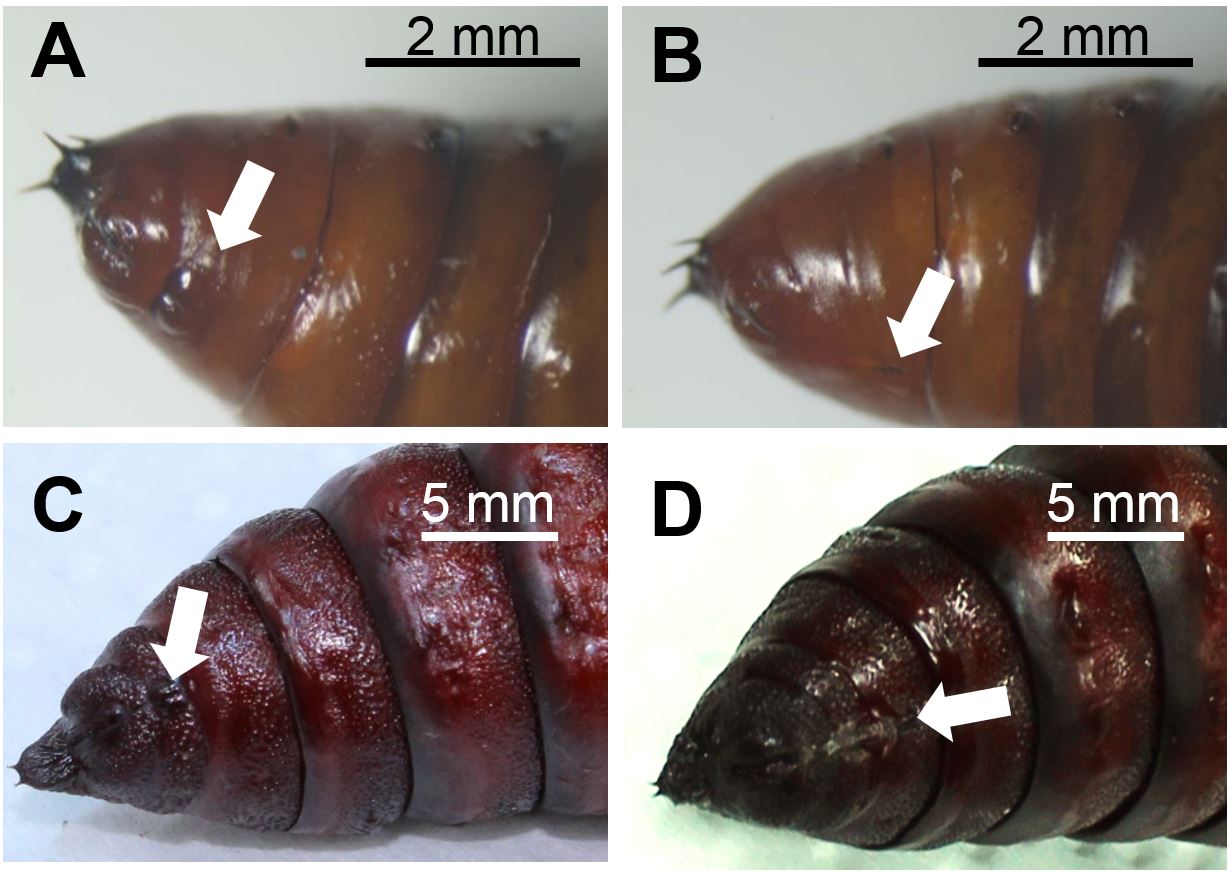
Figure 1. Abdomen of male (A) and female (B) Spodoptera exigua pupae and of male (C) and female (D) Manduca sexta pupae. Arrows indicate genital structures at the ventral side that differ between sexes in morphology and the segments they are located. Photographs: Stefanie Luka (A, B), Anke Steppuhn (C, D). Scale bars, 2 mm (A, B), 5 mm (C, D) - To equalise slight differences in plant ontogeny between treatment groups, sort 4-5 weeks old Nicotiana attenuata rosette stage plants [grown as described in Krügel et al. (2002); Figure 2A] by size (i.e. rosette diameter) and elongation state (degree of stalk development) and assign equal plants to all treatments of each biological replicate. For a full-factorial experimental design that enables to evaluate effects of oviposition on plant defence parameter four treatment groups are required (Box 1).
Box1. Treatments required for a full-factorial priming experimentI. both oviposition and larval feeding after the natural egg incubation time II. oviposition not followed by larval feeding III. larval feeding without prior oviposition IV. control plants without oviposition and larval feeding - To control for the experimental procedure and other stimuli associated with exposure to moths use cages with only male moths for treatments without oviposition (30 male S. exigua moths or 16 male M. sexta moths).
Exposure of N. attenuata to S. exigua oviposition- Whole N. attenuata plants are exposed in the flight cages to oviposition by the mated S. exigua moths (Figure 2B-C). Plants are placed individually in the cage by briefly opening it while taking care that moths do not escape.
Note: S. exigua moths do not lay eggs on defined leaves of N. attenuata, however the moth oviposition behaviour depends on the plant species. Oviposition by S. exigua on defined leaf positions is possible, for example, on Solanum dulcamara plants of which selected leaves are exposed through slots at the cage sides as described below (see “Exposure of N. attenuata to M. sexta oviposition”). - Overnight females lay eggs on the plants, but also on the pots and at the cage (Figure 2D-E). At the next morning, plants can be removed again individually by briefly opening the cage while taking care that the moths do not escape.
Note: To place plants into the flight cages and remove them, a light should be placed behind the cage to attract the moths to the back side of the cage. As the moths may also remain on the plants and pots, at first the plant should be examined for moths, which are returned into the cage. - All leaves are examined for egg clutches and single eggs (Figure 2F). Egg-laden leaves are labelled with hanging labels or a thread. The number of egg clutches, eggs and the leaf position where they are should be documented to first ensure that all eggs are removed before larval hatching and second to be able to explore the effect of these parameters on the larval performance and plant defence expression [see Bandoly and Steppuhn (2015)].
- All non-plant surfaces, i.e., pots and soil, need to be carefully inspected for eggs which are removed with a slightly moistened brush and may be collected in rearing boxes to retrieve larvae e.g., for rearing purposes.
- The time point of hatching can be estimated by regularly examining the eggs for darkening because the head capsule of the larvae starts to melanise shortly before they hatch (Figure 2G). Larvae will hatch after 2-4 d depending on the abiotic conditions in the greenhouse [most importantly temperature, e.g., it takes about 4 days at 25/15 °C (day/night)].
- Shortly before the larvae hatch, remove the eggs gently with a moistened brush to enable a standardised onset and extent of larval feeding as well as to ensure the treatment of oviposited plants without larval feeding. Again the eggs can be collected for further use e.g., for the larval feeding treatments.
Note: Be careful not to damage the plant surface! If the egg attachment is very strong, try to loosen the eggs by applying water and try again after 1-2 min.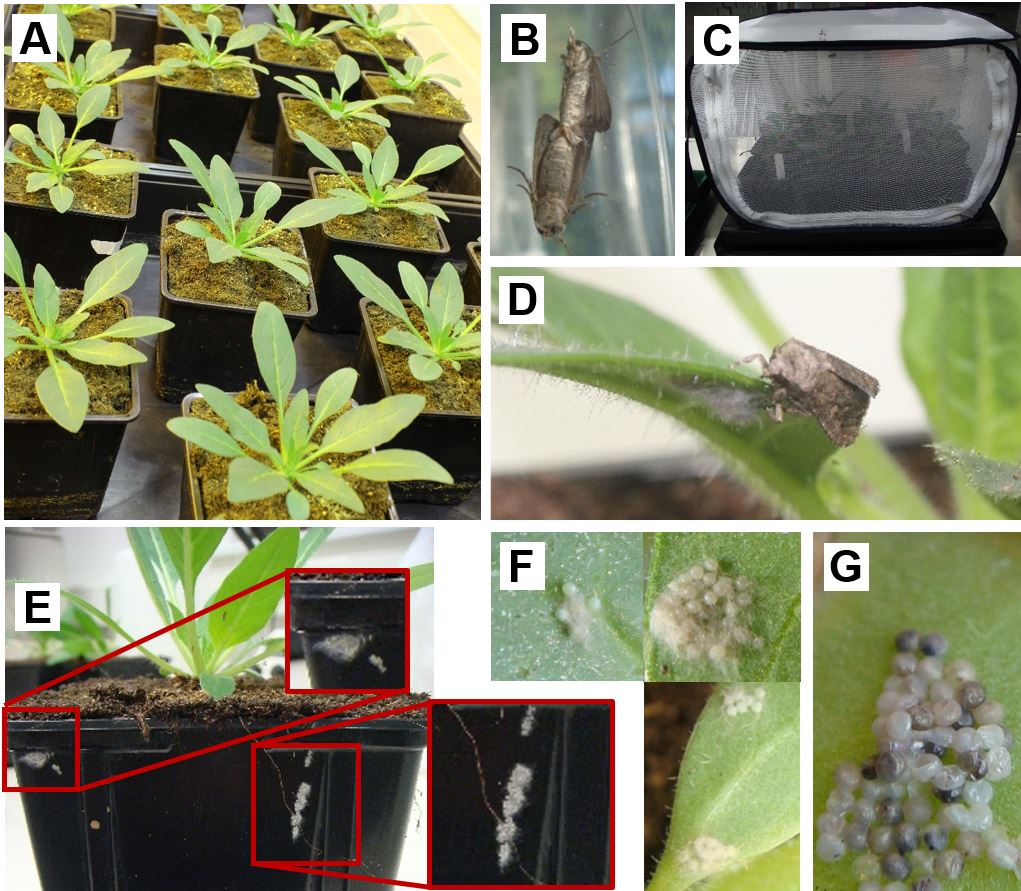
Figure 2. Setup to expose Nicotiana attenuata plants to Spodoptera exigua oviposition. A. Four to five weeks old N. attenuata rosette stage plants. B. Mating S. exigua moths. C. Flight cage with plants and mated moths for oviposition. D. Female moth after oviposition on a leaf. E. Egg clutches on a plant pot. F. Different sizes and numbers of egg clutches on N. attenuata leaves. G. S. exigua eggs turning dark shortly before hatching. Photographs: Michele Bandoly
Exposure of N. attenuata to M. sexta oviposition
Note: As the allocation of defensive compounds is known to depend on leaf ontogeny (van Dam et al., 2001), it is best to standardise the leaf position that is exposed to oviposition, which is possible when the flight cage is prepared as such to bear slots at the sides of a cage in approximately the height that the designated leaf is situated on the plant in its pot (Figure 3A). The edges of the slots should be fixed with a seam so that the netting of the cage will not fray. The slots can be closed with hair clips.- Determine the leaf positions in relation to the sink-source transition leaf (assimilate-import equals assimilate export), which can be estimated according to morphological characteristics as it unifies the characteristics of sink leaves, i.e. a light-green colouration and high trichome density, and that of source leaves, i.e. a dark-green colouration and low trichome density, to about equal extent (Figure 3B).
- Expose the second youngest source leaf of N. attenuata plants (see Figure 3B leaf +2) to M. sexta oviposition by inserting this leaf through slots in the cage that are located around the flight cage (Figure 3A, C).
- To avoid unnatural high egg loads, the oviposition cages have to be observed from dusk onwards, which is when these nocturnal moths become active. Though M. sexta moths lay single or two eggs on a plant under natural conditions, leaf material provided to moths in captivity usually receive overloads of eggs.
Note: The light sources of the greenhouse should be kept off and to observe moth behaviour use a headlamp. - As soon as an oviposition event occurred (Figure 3D), the respective leaf as well as that of the corresponding control plant are removed from the cages.
- Shortly before hatching, which can be estimated from the egg colour turning from light-green to white (Figure 3E), remove the eggs gently with a featherweight forceps. The natural egg incubation time of M. sexta is 4-5 d.
Note: Be careful not to damage the plant surface! If the egg attachment is very strong, try to loosen the eggs by applying water and try again after 1-2 min.
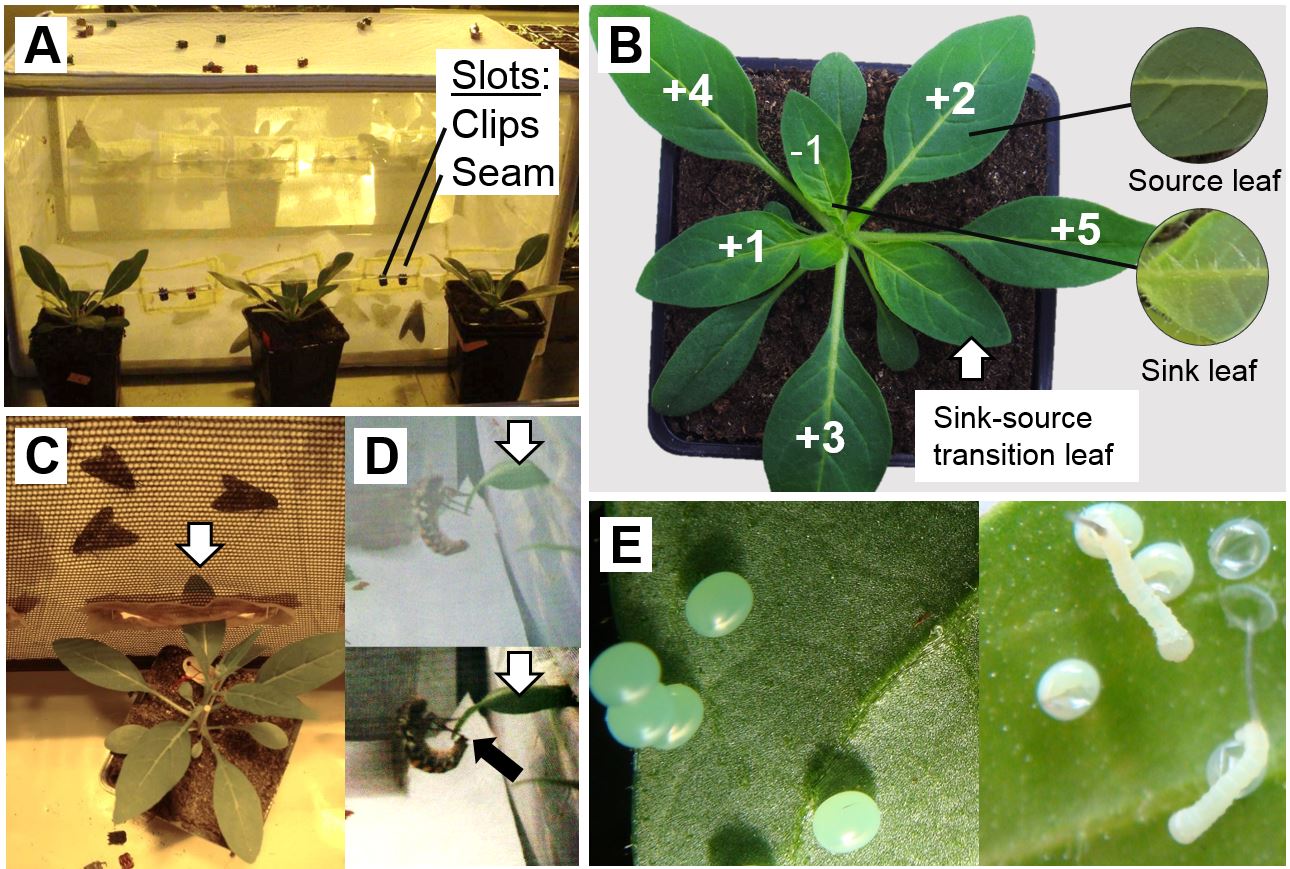
Figure 3. Setup to expose Nicotiana attenuata plants to Manduca sexta oviposition. A. Flight cage with mated M. sexta moths for oviposition on a defined leaf position of N. attenuata rosette stage plants. B. Positions and characteristics of sink and source leaves of N. attenuata in relation to the sink-source transition leaf. C. The +2 leaf (white arrow) is inserted through a slot at the side of the cage. D. Female M. sexta moths approaching the leaf exposed into the cage through a slot (white arrow) and during oviposition (black arrow) on a leaf exposed through a slot into the oviposition cage. E. The initially green M. sexta eggs turn white shortly before the larvae start hatching. Photographs: Michele Bandoly
- Sort S. exigua or M. sexta pupae for their sex. Figure 1 depicts the discrimination characteristics between male and female lepidopteran pupae.
- Larval performance (mortality, mass and developmental time) and feeding damage
- Select a defined leaf position (see Figure 3B) for larval application: either the second youngest source leaf (+2 leaf), a leaf position that is systemic to the leaf oviposited by S. exigua or M. sexta, or the local formerly egg-laden leaf if oviposition occurred on standardised leaves as described for M. sexta.
Note: Due to the plant development during the egg incubation time the leaf positions in relation to the sink-source transition leaf have changed since the day of oviposition. The formerly egg-laden leaf corresponds now to leaf +3 or +4. - Applicate similar numbers of neonate larvae to the selected leaf position of oviposited N. attenuata plants and the corresponding leaf position of control plants.
- As generalist S. exigua larvae feed gregariously and exhibit a high mortality on N. attenuata, apply 15-30 freshly hatched S. exigua larvae (dependent on availability) using a moistened brush (Figure 4A).
- The tobacco specialist M. sexta is feeding solitary or in low number on N. attenuata plants, so that not more than 3 larvae should be applied by grabbing the larvae with a featherweight forceps at their abdominal horn (Figure 4B).
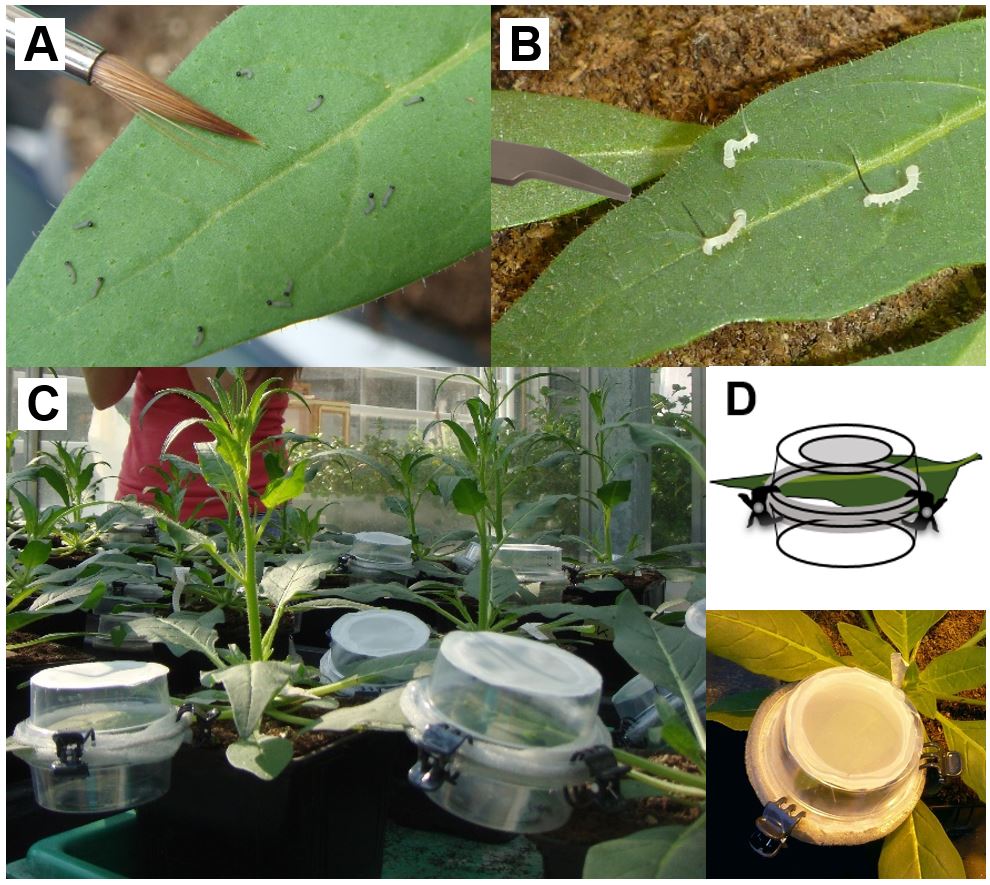
Figure 4. Application of Spodoptera exigua or Manduca sexta neonates. A. S. exigua larvae are applied using a moist brush. B. M. sexta larvae are applied with a featherweight forceps. C, D. Larvae are confined on the selected leaf using vented clip cages. Photographs: Michele Bandoly
- As generalist S. exigua larvae feed gregariously and exhibit a high mortality on N. attenuata, apply 15-30 freshly hatched S. exigua larvae (dependent on availability) using a moistened brush (Figure 4A).
- The larvae are confined on the selected leaf position using vented clip cages (see Figure 4C-D).
- Once per day carefully open the clip cages and examine the leaf as well as the cages for larvae. Count dead and alive larvae to determine larval mortality (in case of S. exigua a folding magnifier may be required to determine whether they are alive).
- To compare developmental times of larvae on oviposited and egg-free control plants also record their developmental stage when examining the larval mortality. In addition to the larval instar, you can also record three sub-instars of each instar to increase resolution (see also Figure 5 and 6A):
- Sub-instar A: Larvae shortly after moulting are characterised by a large head capsule in relation to the body.
- Sub-instar B: Larvae intensively feeding are characterised by a larger body volume in relation to their head capsule.
- Sub-instar C: Larvae preparing to moult are characterised by a tiny head capsule in relation to the body and are often lighter as they stop feeding.
- Sub-instar A: Larvae shortly after moulting are characterised by a large head capsule in relation to the body.
- Larval weight can be measured every 2-3 d (in case of S. exigua, larvae can be first weighted after 3-4 d with a high precision balance of an accuracy of at least 0.1 mg). Again gently transfer small larvae with a moist brush.

Figure 5. Spodoptera exigua larval instars and sub-instars. Freshly moulted (A), larvae in feeding stage (B) and larvae stopped feeding and prepare for moulting (C) are determined from different sizes of the dark brown head capsule in relation to the body diameter. Photographs: Stefanie Luka; Scale bar, 2 mm - To warrant sufficient amounts of leaf material for the larvae to feed, the larvae are moved to the next younger leaf positions if required.
Note: When larvae change instars take into account that larvae feed twice as much than previously. A transfer about every second day can be sufficient during the first week but thereafter larvae have to be transferred daily in longer lasting experiments. - To assess feeding damage caused by the larvae photograph the leaves (without cutting them off) on a whiteboard that is attached to a support stand with clamps that can be adjusted to the required height. The whiteboard should have black reference areas on the four corners. Determine the fed leaf areas from the photographs in a graphic program [e.g., GNU Image Manipulation Program (GIMP) or Photoshop CS5 (Adobe Systems Incorporated, USA)] in relation to the reference areas.
- To assess the antimicrobial activity in the haemolymph of M. sexta larvae as an immune parameter, larvae that were feeding for 6 days on oviposited or control plants are briefly chilled on ice and dabbed with a tissue soaked in 70% ethanol. Then the abdominal horn is cut off with sterilised scissors. The haemolymph is collected with a 10 µl-pipette while leaking out of the wound (Figure 6B). Immediately flash-freeze the haemolymph samples in 0.2 ml tubes in liquid nitrogen and analyse the antimicrobial activity with either a radial diffusion assay or micro-gel well diffusion assay or a microtiter broth dilution method as described in Du Toit and Rautenbach (2000).
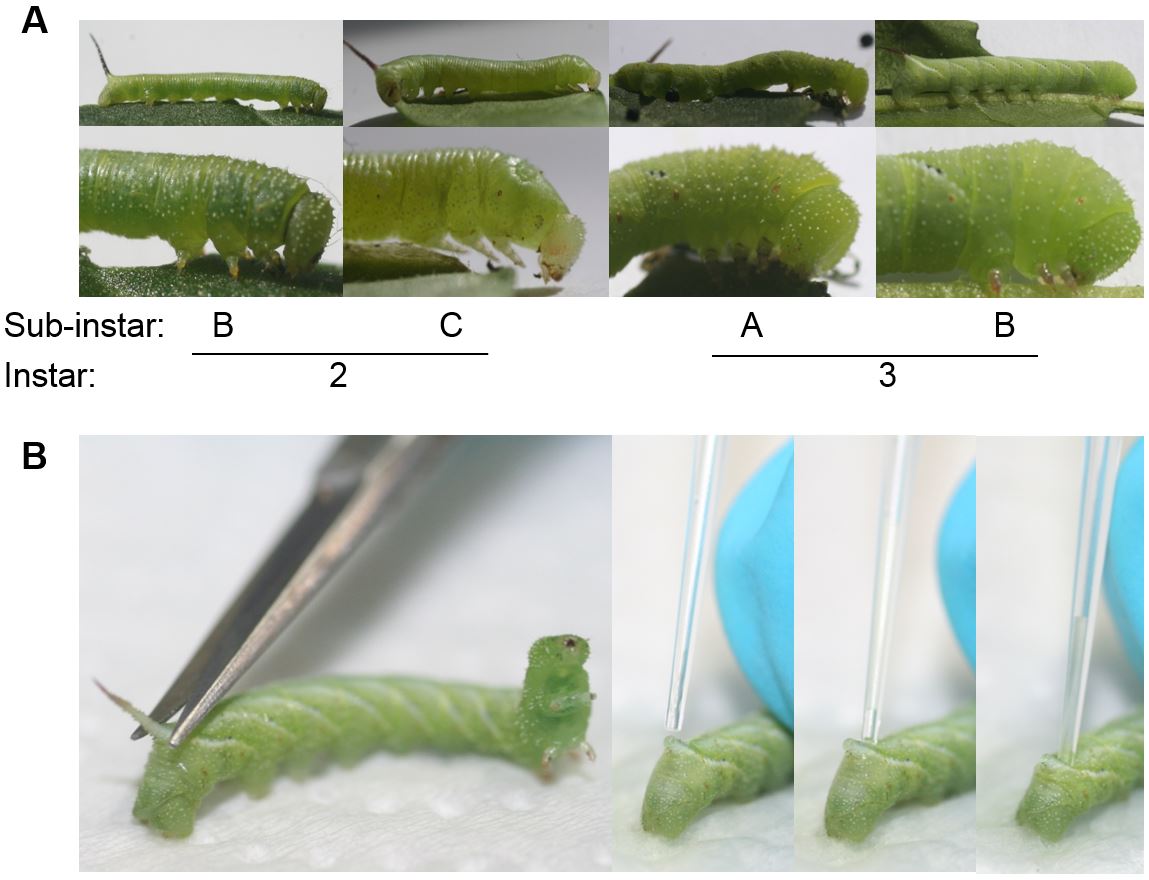
Figure 6. Developmental stages and haemolymph collection of Manduca sexta larvae. A. M. sexta larval instars and sub-instars. (A: Freshly moulted; B: Larvae in feeding stage; C: Larvae stopped feeding and prepare for moulting). B. Collection of haemolymph of young third instar M. sexta larvae from the horn that is cut off using a scissors. Photographs: Anke Steppuhn
- Select a defined leaf position (see Figure 3B) for larval application: either the second youngest source leaf (+2 leaf), a leaf position that is systemic to the leaf oviposited by S. exigua or M. sexta, or the local formerly egg-laden leaf if oviposition occurred on standardised leaves as described for M. sexta.
- Standardised damage and harvest of leaf tissue for analyses of plant defence traits
Note: Feeding-induced defensive compounds of N. attenuata such as caffeoylputrescine and protease inhibitor activity correlate positively with inflicted feeding damage (Bandoly et al., 2015). The feeding damage of S. exigua larvae on previously oviposited plants is lower than on control plants because of an increased larval mortality and a decreased larval growth. Thus, to compare induction levels of these compounds in egg-free and oviposited N. attenuata plants, plants of both treatment groups should be damaged to a comparable extent by either adjusting larval number and size or by mimicked larval herbivory.- Standardisation of feeding damage by S. exigua larvae on oviposited and control N. attenuata plants
- Besides the experimental plants, an additional set oviposited and control plants hosting S. exigua larvae of the same egg batches as used for the experimental plants are used as supplier plants of larvae during the experiment.
- At least once per day experimental plants with and without prior oviposition are evaluated for the number and size of larvae. Differences between oviposited and control plants are adjusted by replacing dead or non-vital larvae with larvae of a corresponding size from the respective supplier plants.
- Ensure that the adjustment indeed resulted in equally damaged plants by determining the feeding damage from photographs as described above.
- Besides the experimental plants, an additional set oviposited and control plants hosting S. exigua larvae of the same egg batches as used for the experimental plants are used as supplier plants of larvae during the experiment.
- Equal damage by mimicked herbivory (repeated wounding and application of larval oral secretions, as previously described e.g., in Halitschke et al., 2001).
- Oral secretions of third instar S. exigua larvae reared on N. attenuata leaf material are collected with a Teflon tube connected to a 4 ml glass vial through the rubber septum in the lid (Figure 7A). The vial is kept on ice and connected through another tube in the lid with a vacuum pump. Provoke regurgitation by the larvae by gently nudging the larvae with the collection tube at the ventral side and collect the oral secretions using a vacuum slightly below atmospheric pressure (Figure 7B). The liquid fraction of the oral secretions after centrifugation in 1.5 ml tubes (at 4 °C) is diluted 1:1 with water for usage. (The oral secretions can be stored for a few days at -20 °C.) Keep oral secretions on ice during usage.
- Inflict a line of puncture wounds to each side of the mid-vein on a selected leaf position (e.g., +2 leaf; see above) of oviposited and egg-free control plants using a pattern wheel (Figure 7C) and immediately add 10 µl along the rows of puncture wounds using a pipette (Figure 7D). Repeat this treatment twice at the same leaves in intervals of 3 h.
- Repeat step C2b with the two next younger source leaves of oviposited and egg-free control plants on the next two days.

Figure 7. Collection of oral secretions from Spodoptera exigua larvae to mimic the larval herbivory by mechanical wounding and application of oral secretions. Oral secretions from larvae are drawn into a glass vial connected to a vacuum pump (A) through a collection tube (B). C. Puncture wounds inflicted with a pattern wheel. D. Application of oral secretions of S. exigua to the wounds. Photographs: Anke Steppuhn (A, B) and Michele Bandoly (C, D) - Oral secretions of third instar S. exigua larvae reared on N. attenuata leaf material are collected with a Teflon tube connected to a 4 ml glass vial through the rubber septum in the lid (Figure 7A). The vial is kept on ice and connected through another tube in the lid with a vacuum pump. Provoke regurgitation by the larvae by gently nudging the larvae with the collection tube at the ventral side and collect the oral secretions using a vacuum slightly below atmospheric pressure (Figure 7B). The liquid fraction of the oral secretions after centrifugation in 1.5 ml tubes (at 4 °C) is diluted 1:1 with water for usage. (The oral secretions can be stored for a few days at -20 °C.) Keep oral secretions on ice during usage.
- Harvest of leaf tissue
- To analyse plant defence compounds harvest the leaves exposed to herbivory or corresponding leaves of control plants (treatment II, IV in Box 1) with a scalpel and immediately flash-freeze the samples in 1.5 ml or 2 ml tubes (use a dissecting forceps to get the leaf material into the tube) in liquid nitrogen. Dependent on the analysed parameter, samples are taken within the first hours or days after herbivory treatment started. To assess the induction kinetics the same leaves may be repeatedly samples by harvesting parts of the leaves from the tip to the base as described [see Bandoly et al. (2015)].
- Analyse N. attenuata defence related compounds and regulators.
- Alkaloids and phenolics by high performance liquid chromatography (HPLC) according to Keinänen et al. (2001) 2-6 d after onset of herbivory.
- Protease inhibitor activity by a radial diffusion assay or a photometric assay in microwell plates as for example described in van Dam et al. (2001) and in Bandoly et al. (2015) 2-6 d after onset of herbivory.
- Phytohormones by liquid chromatography coupled to mass spectrometry (LC-MS) as for example described in Gaquerel et al. (2012) within the first hours after onset of herbivory.
- Defence gene expression by real-time qPCR as for example described in Bandoly et al. (2015) at a time point the respective gene is induced.
- Alkaloids and phenolics by high performance liquid chromatography (HPLC) according to Keinänen et al. (2001) 2-6 d after onset of herbivory.
- To analyse plant defence compounds harvest the leaves exposed to herbivory or corresponding leaves of control plants (treatment II, IV in Box 1) with a scalpel and immediately flash-freeze the samples in 1.5 ml or 2 ml tubes (use a dissecting forceps to get the leaf material into the tube) in liquid nitrogen. Dependent on the analysed parameter, samples are taken within the first hours or days after herbivory treatment started. To assess the induction kinetics the same leaves may be repeatedly samples by harvesting parts of the leaves from the tip to the base as described [see Bandoly et al. (2015)].
- Standardisation of feeding damage by S. exigua larvae on oviposited and control N. attenuata plants
Data analysis
Example data for the effects of oviposition on the larvae, their feeding damage and the induction of plant defence parameters in the study systems described here as well as a statistical analyses of these data are presented in Bandoly et al. (2015) and Bandoly et al. (2016).
Acknowledgments
We would like to thank Roland Grichnik for aiding in the development of the bioassays with M. sexta described here. We also acknowledge the authors of previous studies that had established for example how to standardise leaf stages and analyse defence metabolites of N. attenuata of which this protocol could only cite examples. We are grateful for the financial support by the German Research Foundation (DFG; project B2 within the Collaborative Research Centre 973) and the German Federal Environmental Foundation (DBU), which supported Michele Bandoly with a stipend.
References
- Bandoly, M. and Steppuhn, A. (2016). A push-button: Spodoptera exigua oviposition on Nicotiana attenuata dose-independently primes the feeding-induced plant defense. Plant Signal Behav 11(1): e1114198.
- Bandoly, M., Grichnik, R., Hilker, M. and Steppuhn, A. (2016). Priming of anti-herbivore defence in Nicotiana attenuata by insect oviposition: herbivore-specific effects. Plant Cell Environ 39(4): 848-859.
- Bandoly, M., Hilker, M. and Steppuhn, A. (2015). Oviposition by Spodoptera exigua on Nicotiana attenuata primes induced plant defence against larval herbivory. Plant J 83(4): 661-672.
- du Toit, E. A. and Rautenbach, M. (2000). A sensitive standardised micro-gel well diffusion assay for the determination of antimicrobial activity. J Microbiol Methods 42(2): 159-165.
- Gaquerel, E., Steppuhn, A. and Baldwin, I. T. (2012). Nicotiana attenuata alpha-DIOXYGENASE1 through its production of 2-hydroxylinolenic acid is required for intact plant defense expression against attack from Manduca sexta larvae. New Phytol 196(2): 574-585.
- Halitschke, R., Schittko, U., Pohnert, G., Boland, W. and Baldwin, I.T. (2001). Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125: 711-717.
- Hilker, M., Schwachtje, J., Baier, M., Balazadeh, S., Baurle, I., Geiselhardt, S., Hincha, D. K., Kunze, R., Mueller-Roeber, B., Rillig, M. C., Rolff, J., Romeis, T., Schmulling, T., Steppuhn, A., van Dongen, J., Whitcomb, S. J., Wurst, S., Zuther, E. and Kopka, J. (2015). Priming and memory of stress responses in organisms lacking a nervous system. Biol Rev Camb Philos Soc.
- Keinanen, M., Oldham, N. J. and Baldwin, I. T. (2001). Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J Agric Food Chem 49(8): 3553-3558.
- Krügel, T., Lim, M., Gase, K., Halitschke, R. and Baldwin, I.T. (2002). Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12:177-183.
- Schoonhoven, L. M., van Loon, J. A. and Dicke, M. (2005). Insect-plant biology second edition. Oxford University Press. 421 pages (ISBN: 9780198525950)
- van Dam, N. M., Horn, M., Mares, M. and Baldwin, I. T. (2001). Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27(3): 547-568.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bandoly, M. and Steppuhn, A. (2016). Bioassays to Investigate the Effects of Insect Oviposition on a Plant’s Resistance to Herbivores. Bio-protocol 6(11): e1823. DOI: 10.21769/BioProtoc.1823.
Category
Plant Science > Plant physiology > Plant growth
Plant Science > Plant physiology > Biotic stress
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link